A Guide To: Alcoholic Fermentation
By: Brent Nakano
The difference between fermentation and spoilage is the control of the developing microorganisms, selecting those that are desired and reducing or eliminating those that are not. Historically, it was known that temperature and air exposure could affect the production of fermented beverages, and recipes were created to achieve replicable results; however, the understanding of the existence of these microorganisms, how they work and how to better control them is relatively new compared to the multi-thousand year history of fermented alcoholic beverages. This research continues to evolve with advances in science.
Foundational History of Yeast Research
The history of yeast and microbial research is generally linked to making better beer and wine as well as advancements in microscopy. Additionally, yeast, particularly Saccharomyces Cerevisiae, has been studied to better understand eukaryotic organisms (organisms with a membrane-bound nucleus as opposed to no-nucleus prokaryotic cells, like bacteria). For this reason, there is a tremendous amount of academic work published on the topic. Dr. James Barnett, in his [14] essay collection of a Historical overview of yeast research, included the following key moments in the academic work starting with
|
1665 to approximately 1678:
Advances were made in microscopy by Antoni van Leeuwenhoek and Robert Hooke to observe microorganisms [2]. 1789: French chemist Antoine Lavoisier studies the production of wine and through a series of analyses. He then published the observed chemical changes during fermentation. This includes:
1803: The Institut de France offered a medal, valued at one kilogram of gold, to answer the question: “What are the characteristics which distinguish vegetable and animal substances acting as ferments from those that undergo fermentation?” 1815: Gay-Lussac revises the proportions of Lavoisier’s findings. According to James Barnett, the alcoholic fermentation equation is often misattributed to Gay-Lussac’s 1815 paper however, the empirical formula for glucose was not established until Dumas (1843), Gay-Lussac died in 1850, and the formal molecular formula was not developed until Baeyer (1870) and Fittig (1871). 1835: Charles Cagniard de la Tour, a physicist and engineer of Paris, observed beer and wine yeast during fermentation and their breakdown of sugar into a spirituous liquor and their release of CO2. Furthermore, he described in his observations that:
1837:
The decomposition must occurs when the sugar-fungus uses sugar and nitrogenous substances for growth. The unused elements are preferentially converted to alcohol. Alcoholic fermentation and putrefaction are not caused by oxygen (as previously suggested by Gay-Lussac), but rather something in the air which can be destroyed by heat. In 1839, Schwann developed “cell theory” which posits that living structures originate from the formation and differentiation of units (the cells), which then constitute the bodies of organisms. 1839 to 1850s: Schwann findings were dismissed by some of the leading chemists as they believed yeast were a physicochemical phenomenon. This may have occurred as organic chemistry and the synthesis of organic compounds from inorganic sources (for example urea from ammonium cyanate by Wohler in 1828), were at the forefront of their research. 1850s to 1880s: As yeast become accepted as the cause of alcoholic fermentation and as microbes after different kinds of yeast and bacteria are studied, the primary question becomes: ”Is fermentation attributed to the intracellular activities or to extracellular enzymes?” |
1857:
In his first paper on alcoholic fermentation, French chemist, Louis Pasteur, invalidates the catalytic theory, citing that if the reaction was catalyzed, nothing would be extracted or removed from the fermentable material; thus, everything should weigh the same at the start and end of the process. Fermentation showed yeast extracting something from the sugar; Pasteur associated the breakdown of sugar into alcohol and carbonic acid with living processes and sugar providing part of the material of the yeast. 1858 and 1859: When consulting with an alcohol producer who was using beet sugar as the fermentable base during the Napoleonic Wars, Pasteur found that the fermentation produced lactic acid and that the globules were round when the fermentation was satisfactory and became elongated as the globules deteriorated. Pasteur also found that the lactic acid cells contained “much smaller cells than the yeast,” showing two different types of fermentation and fermentation microbes. 1860: Pasteur concluded
1863: The terms aerobic and anaerobic came into being. An English translation of Pasteur's work: Pasteur, L. (2016, May 8). Physiological Theory of Fermentation. Pasteur Brewing. Retrieved February 25, 2022, from www.pasteurbrewing.com/physiological-theory-of-fermentation/ 1877: Emil Fischer produced phenylhydrazine, which helped to reveal the molecular structure of sugar and other nitrogen compounds [4]. 1897: Through the use of yeast extract, Eduard Buchner showed that the biochemical process does not require living cells, but rather the cell’s constituents which include enzymes [5]. 1905: Arthur Harden and William Young found that fermentation required inorganic phosphate and that it required the presence of both a heat-labile component they called “zymase” and a heat-stable fraction, with a low molecular weight, called “cozymase.” 1929: Karl Lohmann, Yellapragada Subbarao, and Cirus Friske independently discovered Adenosine Triphosphate (ATP), the molecule used by enzymes and other proteins in many cellular processes [6]. 1940: The metabolic pathways of glycolysis were linked together by Otto Fritz Meyerhof using the metabolic process of his predecessors, including Pasteur, Buchner, Harden, and others [7]. 1950s:
|
For additional insight into genetic mapping of yeast read:
Steve Swinnen, Johan M Thevelein, Elke Nevoigt, Genetic mapping of quantitative phenotypic traits in Saccharomyces cerevisiae, FEMS Yeast Research, Volume 12, Issue 2, March 2012, Pages 215–227, https://doi.org/10.1111/j.1567-1364.2011.00777.x
For an in-depth history of yeast research by Dr. James Barnett, read the 14 essay collection on yeast originally published in the Academic Journal:
Barnett, J. A., & Barnett, L. (2011). Yeast research: a historical overview. American Society for Microbiology Press
Steve Swinnen, Johan M Thevelein, Elke Nevoigt, Genetic mapping of quantitative phenotypic traits in Saccharomyces cerevisiae, FEMS Yeast Research, Volume 12, Issue 2, March 2012, Pages 215–227, https://doi.org/10.1111/j.1567-1364.2011.00777.x
For an in-depth history of yeast research by Dr. James Barnett, read the 14 essay collection on yeast originally published in the Academic Journal:
Barnett, J. A., & Barnett, L. (2011). Yeast research: a historical overview. American Society for Microbiology Press
Yeast Production of Flavor
Fermentable sugars
Monosaccharides: A class of sugars that cannot be further broken down into simpler sugars.
In pre-fermentation processes, non-monosaccharide sugars need to be converted into glucose or fructose in order to be metabolized by yeast into ethanol.
Galactose, a monosaccharide, is rarely broken down by yeast because it requires additional enzymes for metabolism. Sources of galactose include: dairy products, avocados, sugar beets.
Disacchrides
Trisaccharides
Polysaccharides: Long chains of monosaccharides.
Monosaccharides: A class of sugars that cannot be further broken down into simpler sugars.
- Glucose, the ideal sugar that yeast consume
- Fructose (however, glucose is preferred by yeast).
In pre-fermentation processes, non-monosaccharide sugars need to be converted into glucose or fructose in order to be metabolized by yeast into ethanol.
Galactose, a monosaccharide, is rarely broken down by yeast because it requires additional enzymes for metabolism. Sources of galactose include: dairy products, avocados, sugar beets.
Disacchrides
- Sucrose is converted into fructose and glucose by yeast through the secretion of the invertase enzyme. Examples include: sugarcane, sugar beets, maple syrup, dates, and honey
- Maltose is converted into 2x glucose by the maltase enzyme. Examples include: The primary sugar in beer wort and is derived from the breakdown of starch by amylase enzymes during mashing.
- Lactose is galactose and glucose broken down by the enzyme lactase.
Trisaccharides
- Maltotriose is converted into 3x glucose and is typically the last sugar to be fermented in brewing wort.
Polysaccharides: Long chains of monosaccharides.
- Starch: A polysaccharide present in the malt which is broken down by the enzyme beta amylase into maltose. This makes up approximately 40% of the wort’s total carbohydrate content.
- Fructans: These are long chains of fructose. In agave fermentation, for example, fructose is hydrolyzed (broken down) by cooking or with an acid.
- Dextrin, lactose, can be added to beer and add mouthfeel without adding additional fermentable sugar.
|
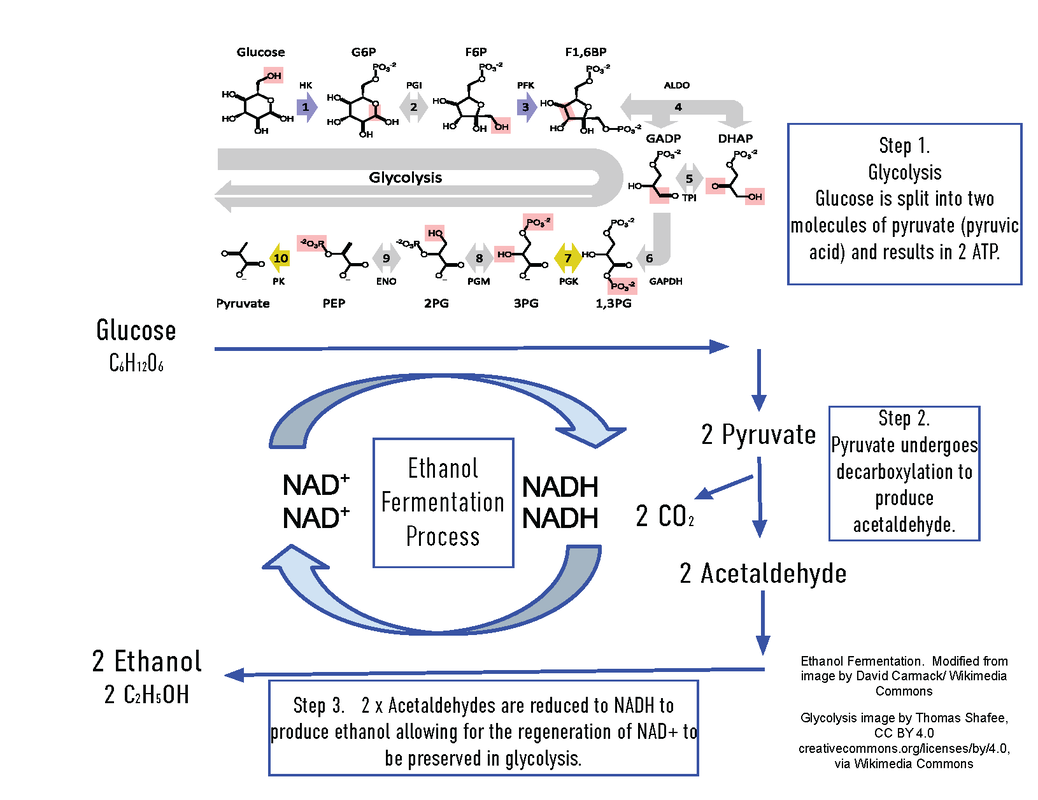
Ethanol Fermentation
Aroma Notes
Process Notes
|
Other Aromatic compounds
Yeast, like many other organisms, use a multitude of metabolic pathways to synthesize necessary components required for growth. The particular aroma compounds are often unique to whatever is being fermented. For more on yeast metabolism read: Pfeiffer, T., & Morley, A. (2014). An evolutionary perspective on the Crabtree effect. Frontiers in molecular biosciences, 1, 17. https://doi.org/10.3389/fmolb.2014.00017 |
Yeast Growth Phases
In batch-fermented wine, nutrients are more available at the beginning of fermentation then declines until most sugars have been metabolized, leaving the wine “dry.” The growth pattern phases are: lag, log, stationary, and decline. The duration of these phases vary by the type of yeast used.
Lag
Immediately following inoculation, cells adjust to the new environment. As some cells do not acclimate successfully, there is an initial period in which the number of new cells produced approximates the number that die.
Log (logarithmic)
Yeast cells generally multiply at a logarithmic (exponential) and consistent rate until the conditions become unfavorable (and they reach their maximum quantity).
Stationary
Yeast cell growth occurs at the same rate at which yeast die making the yeast population “stationary”. During this phase nutrient content declines and metabolic byproducts toxic to yeast (but desirable to flavor) accumulate.
Decline
The yeast population declines as more cells die then divide. This occurs as nutrients continue to decline and the concentration of toxic metabolites continues to increase. Cells are not replaced, the colony eventually perishes, or becomes dormant.
Lag
Immediately following inoculation, cells adjust to the new environment. As some cells do not acclimate successfully, there is an initial period in which the number of new cells produced approximates the number that die.
Log (logarithmic)
Yeast cells generally multiply at a logarithmic (exponential) and consistent rate until the conditions become unfavorable (and they reach their maximum quantity).
Stationary
Yeast cell growth occurs at the same rate at which yeast die making the yeast population “stationary”. During this phase nutrient content declines and metabolic byproducts toxic to yeast (but desirable to flavor) accumulate.
Decline
The yeast population declines as more cells die then divide. This occurs as nutrients continue to decline and the concentration of toxic metabolites continues to increase. Cells are not replaced, the colony eventually perishes, or becomes dormant.
Sources and Suggested Reading
1.Barnett, J. A. (2003). Beginnings of microbiology and biochemistry: the contribution of yeast research. Microbiology, 149(3), 557-567. http://rpdata.caltech.edu/courses/PBoC%20GIST%202012/files_2012/articles/Articles%20added%20during%20class/Barnett%202003%20Beginnings%20of%20microbiology%20and%20biochemistry.pdf
2.Gest, H. (2004). The discovery of microorganisms by Robert Hooke and Antoni Van Leeuwenhoek, fellows of the Royal Society. Notes and records of the Royal Society of London, 58(2), 187-201. Source: https://royalsocietypublishing.org/doi/abs/10.1098/rsnr.2004.0055 https://backyardbrains.com/experiments/files/2004_Gest_The%20discovery_of_microorganisms.pdf
3.Barnett, J. A. (1998). A history of research on yeasts 1: work by chemists and biologists 1789–1850. Yeast, 14(16), 1439-1451. https://doi.org/10.1002/(SICI)1097-0061(199812)14:16<1439::AID-YEA339>3.0.CO;2-Z
4.NobelPrize.org. Nobel Prize Outreach AB 2022. Emil Fischer – Facts. Thu. 24 Feb 2022. https://www.nobelprize.org/prizes/chemistry/1902/fischer/facts
5.NobelPrize.org. Nobel Prize Outreach AB 2022. Eduard Buchner – Facts. Thu. 24 Feb 2022. https://www.nobelprize.org/prizes/chemistry/1907/buchner/facts/
6.Alba-Lois, L., & Segal-Kischinevzky, C. (2010). Yeast fermentation and the making of beer and wine. Nature Education, 3(9), 17. www.nature.com/scitable/topicpage/yeast-fermentation-and-the-making-of-beer-14372813/
7.Kresge, N., Simoni, R. D., & Hill, R. L. (2005). Otto Fritz Meyerhof and the elucidation of the glycolytic pathway. Journal of Biological Chemistry, 280(4), e3. https://doi.org/10.1016/S0021-9258(20)76366-0
8.Lallemand. (n.d.). THE FERMENTATION OF FRUCTOSE IN WINEMAKING. Lallemand Wine. Retrieved February 20, 2022, from www.lallemandwine.com/wp-content/uploads/2014/12/Wine-Expert-The-Fermentation-of-Fructose-in-winemaking-2013.pdf
9.Wikipedia. (n.d.). Ethanol fermentation. Wikipedia. Retrieved February 24, 2022, from https://en.wikipedia.org/wiki/Ethanol_fermentation
The Good Scents Company. (n.d.). Pyruvic Acid, 127-17-3. The Good Scents Company. Retrieved February 25, 2022, from www.thegoodscentscompany.com/data/rw1034261.html
2.Gest, H. (2004). The discovery of microorganisms by Robert Hooke and Antoni Van Leeuwenhoek, fellows of the Royal Society. Notes and records of the Royal Society of London, 58(2), 187-201. Source: https://royalsocietypublishing.org/doi/abs/10.1098/rsnr.2004.0055 https://backyardbrains.com/experiments/files/2004_Gest_The%20discovery_of_microorganisms.pdf
3.Barnett, J. A. (1998). A history of research on yeasts 1: work by chemists and biologists 1789–1850. Yeast, 14(16), 1439-1451. https://doi.org/10.1002/(SICI)1097-0061(199812)14:16<1439::AID-YEA339>3.0.CO;2-Z
4.NobelPrize.org. Nobel Prize Outreach AB 2022. Emil Fischer – Facts. Thu. 24 Feb 2022. https://www.nobelprize.org/prizes/chemistry/1902/fischer/facts
5.NobelPrize.org. Nobel Prize Outreach AB 2022. Eduard Buchner – Facts. Thu. 24 Feb 2022. https://www.nobelprize.org/prizes/chemistry/1907/buchner/facts/
6.Alba-Lois, L., & Segal-Kischinevzky, C. (2010). Yeast fermentation and the making of beer and wine. Nature Education, 3(9), 17. www.nature.com/scitable/topicpage/yeast-fermentation-and-the-making-of-beer-14372813/
7.Kresge, N., Simoni, R. D., & Hill, R. L. (2005). Otto Fritz Meyerhof and the elucidation of the glycolytic pathway. Journal of Biological Chemistry, 280(4), e3. https://doi.org/10.1016/S0021-9258(20)76366-0
8.Lallemand. (n.d.). THE FERMENTATION OF FRUCTOSE IN WINEMAKING. Lallemand Wine. Retrieved February 20, 2022, from www.lallemandwine.com/wp-content/uploads/2014/12/Wine-Expert-The-Fermentation-of-Fructose-in-winemaking-2013.pdf
9.Wikipedia. (n.d.). Ethanol fermentation. Wikipedia. Retrieved February 24, 2022, from https://en.wikipedia.org/wiki/Ethanol_fermentation
The Good Scents Company. (n.d.). Pyruvic Acid, 127-17-3. The Good Scents Company. Retrieved February 25, 2022, from www.thegoodscentscompany.com/data/rw1034261.html