Post-Fermentation Processes: Stabilization
By: Brent Nakano
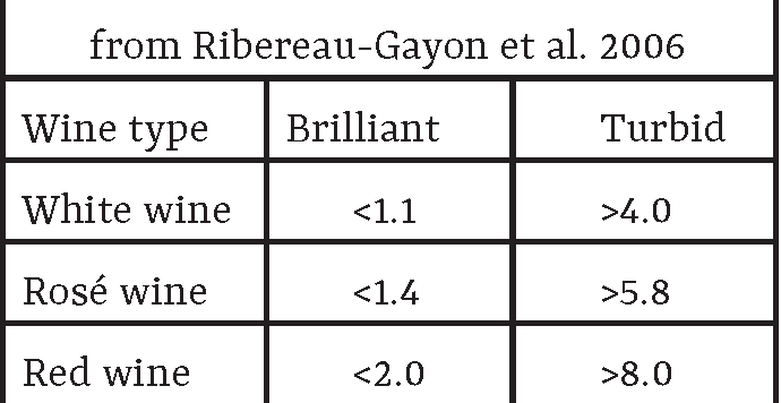
There are a multitude of processes after fermentation which ensure that bottled wine does not change in an undesired way. Typically, these steps come after barrel-aging and any aroma adjustments. The general processes involved are fining, clarification, and filtration. However, depending on the technique used, the flavor of the wine can be modified. The following are many common options for stabilizing wine and their potential impact on wine aroma.
For this piece, we started by referencing Ronald Jackson’s Wine Science, a textbook we highly recommend for those interested in learning about wine production. It can be purchased here: https://www.elsevier.com/books/wine-science/jackson/978-0-12-816118-0
For this piece, we started by referencing Ronald Jackson’s Wine Science, a textbook we highly recommend for those interested in learning about wine production. It can be purchased here: https://www.elsevier.com/books/wine-science/jackson/978-0-12-816118-0