Wine Polyphenols
By: Brent Nakano
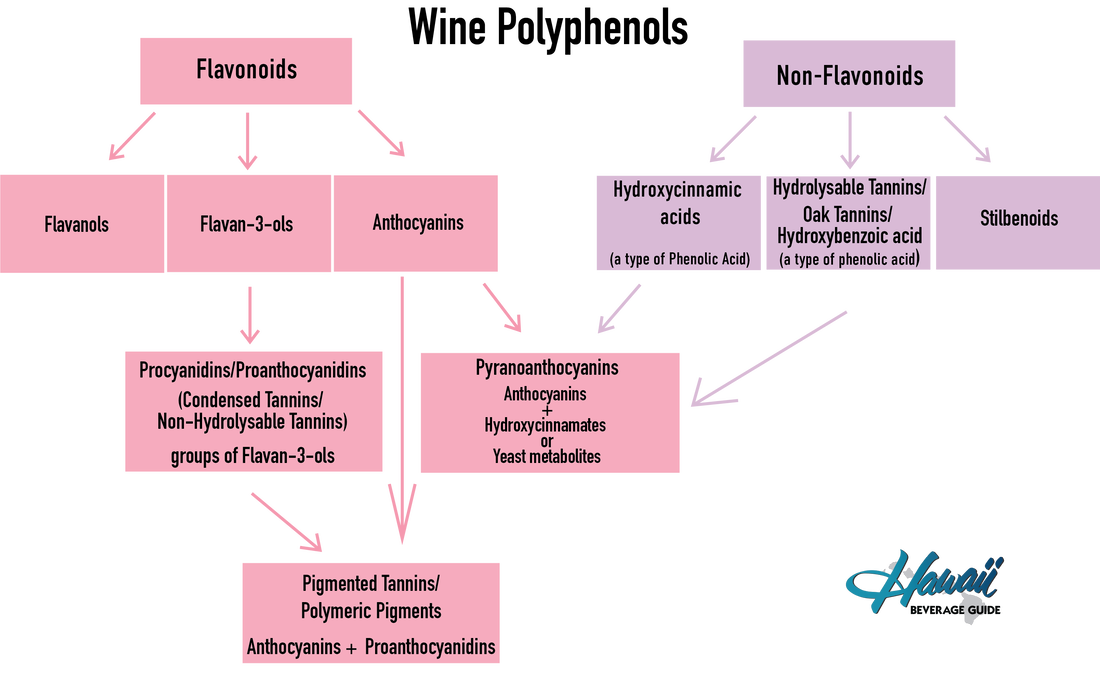
Phenols are a benzene ring attached to one or more hydroxyl groups. In nature, these phenols occur in groups called polyphenols. The polyphenols in wine are predominantly derived from grape skins, and seeds with minor contributions from oak or other wood. Their general accumulation during grape berry development is driven by ripeness, to the extent that it is one of the two general measures of ripeness, the other being sugar ripeness. Polyphenols are not just tannins and anthocyanins, however, but rather a diverse set of compounds that impact mouthfeel and color. In general, these can be divided into non-flavonoid phenols and flavonoid phenols.
For this Guide we referenced the following overview articles that we suggest reading:
Ronald Jackson’s Wine Science, our favorite Wine Textbook, which can be purchased at www.elsevier.com/books/wine-science/jackson/978-0-12-816118-01
Virginia Tech’s Wine/ Enology Grape Chemistry Group Winemaking Topics and in particular the Red Wine Production Considerations by Dr. Bruce Zockelein
www.apps.fst.vt.edu/extension/enology/winemakingissues.html
Jennifer Angelosante’s A Guide to Wine Phenolics on GuildSomm
www.guildsomm.com/public_content/features/articles/b/
jennifer-angelosante/posts/phenolics2
Sabrina Leuck's 2020 youtube lecture for the Institute for Enology & Viticulture at Walla Walla Community College Introduction to Wine Phenolics www.youtube.com/watch?v=uIJUh8nma-E 39
For this Guide we referenced the following overview articles that we suggest reading:
Ronald Jackson’s Wine Science, our favorite Wine Textbook, which can be purchased at www.elsevier.com/books/wine-science/jackson/978-0-12-816118-01
Virginia Tech’s Wine/ Enology Grape Chemistry Group Winemaking Topics and in particular the Red Wine Production Considerations by Dr. Bruce Zockelein
www.apps.fst.vt.edu/extension/enology/winemakingissues.html
Jennifer Angelosante’s A Guide to Wine Phenolics on GuildSomm
www.guildsomm.com/public_content/features/articles/b/
jennifer-angelosante/posts/phenolics2
Sabrina Leuck's 2020 youtube lecture for the Institute for Enology & Viticulture at Walla Walla Community College Introduction to Wine Phenolics www.youtube.com/watch?v=uIJUh8nma-E 39