- Subscribe
- Digital Edition
-
Beverage Guide
- Flavor and Cocktail Construction >
- Production Fundamentals >
- Non-Alcoholic Beverages >
-
Beer
>
- Cider >
- Sake
-
Spirits
>
-
Wine
>
- Kamaʻāina Wine Makers >
-
Winemaking
>
- A Guide to: High Sugar Residual Wines
- A Guide to: Post Fermentation Flavor Adjustments
- A Guide to: Post Fermentation Process: Stabilization
- A Guide to: Wine Prefermentation Practices
- A Guide to: Wine Microbes
- A Guide to: Wine Alcoholic Fermentation Physical Environment
- A Guide to: Wine Fermentation Chemical Environment
- A Guide to: Wine Bottling
- A Guide to: Wine Faults
- A Guide to: Wine Polyphenols
- A Guide to: Wine Aroma Compounds: Pt 1
- A Guide to: Wine Aroma Compounds: Pt 2
- A Guide to Viticulture
- Red and White Grape Aroma Compounds
- Wine Styles >
- Business Strategy
- News and Events
- About
- Production Fundamentals
- Flower Aroma Compounds
- Flavor Pairing and Recipe Development
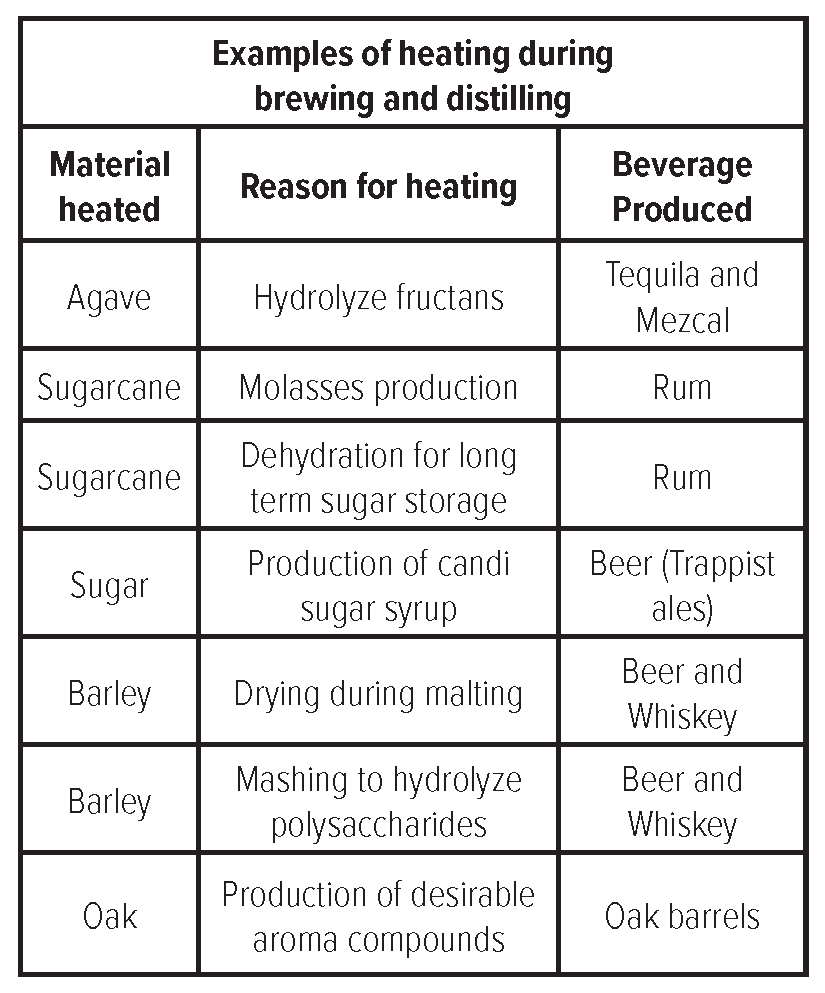
Heat is applied during brewing and distilling for dehydration of fermentable materials, the conversion
of complex sugars into fermentable ones, and the production of desirable aroma compounds. Like in many
foods including bread and coffee, this application of heat produces a brown color with toasty, roasty flavors.
These flavors are caused by heat induced non-enzymatic browning reactions including pyrolysis reactions,
Maillard reactions, and Strecker Degradation.
Additionally, many of the resulting aromatic compounds have multiple production pathways. For this article we have summarized the following literature reviews which we highly recommend reading in their entirety:
[1] Corzo-Martínez, M., Corzo, N., Villamiel, M., & Del Castillo, M. D.
(2012). Browning reactions. Foodbiochemistry and food processing, 56-83.
Retrieved from: www.academia.edu/33958572/Browning_Reactions
[2] Martins, S., Jongen, W. M. F., van Boekel, M. (2000). A review
of Maillard reaction in food and implications to kinetic modeling,
Trends Food Sci. Tech. 11, 364-373.
https://ucanr.edu/datastoreFiles/608-648.pdf
[3] Coghe, S., Derdelinckx, G., & Delvaux, F. R. (2004). Effect of
nonenzymatic browning on flavour, colour and antioxidative activity
of dark specialty malts—a review. Monatsschr. Brauwiss, 57, 25-38.
Retrieved from: http://themodernbrewhouse.com/wp-content/uploads/2017/04/
Coghe0604.pdf
[4] Gernat, D.C., Brouwer, E. & Ottens, M. (2020). Aldehydes as Wort
Off-Flavours in Alcohol-Free Beers—Origin and Control. Food Bioprocess
Technol 13, 195–216. https://doi.org/10.1007/s11947-019-02374-z
of complex sugars into fermentable ones, and the production of desirable aroma compounds. Like in many
foods including bread and coffee, this application of heat produces a brown color with toasty, roasty flavors.
These flavors are caused by heat induced non-enzymatic browning reactions including pyrolysis reactions,
Maillard reactions, and Strecker Degradation.
Additionally, many of the resulting aromatic compounds have multiple production pathways. For this article we have summarized the following literature reviews which we highly recommend reading in their entirety:
[1] Corzo-Martínez, M., Corzo, N., Villamiel, M., & Del Castillo, M. D.
(2012). Browning reactions. Foodbiochemistry and food processing, 56-83.
Retrieved from: www.academia.edu/33958572/Browning_Reactions
[2] Martins, S., Jongen, W. M. F., van Boekel, M. (2000). A review
of Maillard reaction in food and implications to kinetic modeling,
Trends Food Sci. Tech. 11, 364-373.
https://ucanr.edu/datastoreFiles/608-648.pdf
[3] Coghe, S., Derdelinckx, G., & Delvaux, F. R. (2004). Effect of
nonenzymatic browning on flavour, colour and antioxidative activity
of dark specialty malts—a review. Monatsschr. Brauwiss, 57, 25-38.
Retrieved from: http://themodernbrewhouse.com/wp-content/uploads/2017/04/
Coghe0604.pdf
[4] Gernat, D.C., Brouwer, E. & Ottens, M. (2020). Aldehydes as Wort
Off-Flavours in Alcohol-Free Beers—Origin and Control. Food Bioprocess
Technol 13, 195–216. https://doi.org/10.1007/s11947-019-02374-z
Pyrolysis
|
Derived from the Greek words pyro meaning "fire" or "heat" and lysis meaning "separating",
pyrolysis is a general term to describe the thermal decomposition of a material, often in an inert atmosphere. As it pertains to food and beverage, pyrolysis typically breaks down organic compounds composed of carbon chains, with the reaction mechanisms and reaction parameters varying on the material being broken down. Examples • Pyrolysis of sugar = Caramelization. Occurs at: >105°C. • Pyrolysis of hemicellulose and lignin in oak barrels occurs at 200°F (93.33 °C). For more insight into the heat treatment of oak barrels: www.hawaiibevguide.com/oak-barrels.html. |
|
Chemistry terms to know
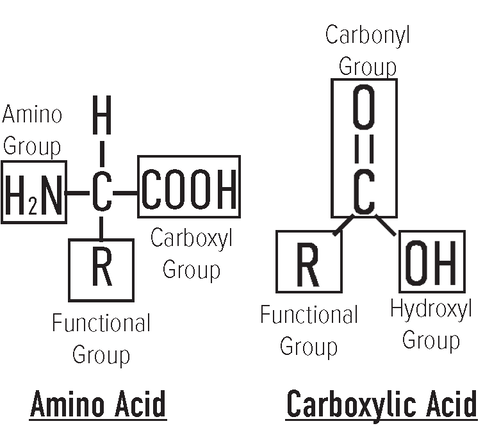
Functional group: In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. Functional groups are represented by: R Hydroxyl group: A hydrogen atom covalently bonded to an oxygen atom. Hydroxyl groups are represented by: -OH Carbonyl group: Carbon atom double-bonded to an oxygen atom. Carbonyl groups are represented by: C=O Aldehyde: A carbonyl group attached to a carbon atom at the end of a carbon chain [6]. Aldehyde groups are represented by −CH=O Carboxylic acid/ Carboxyl Group: An organic compound that contains a carbon double bonded to oxygen and single bonded to hydroxyl group. An additional bond can be attached to a hydrogen or R-group (an alkyl, alkenyl, aryl, or other group) [7]. Examples include amino acids and fatty acids which have chains 16-18 carbons long with a carboxylic group acids attached. Carboxylic acids are represented by: −COOH or −CO2H Imine: A functional group or compound containing a carbon–nitrogen double bond (N=C). The nitrogen atom may be attached to hydrogen (H) or an organic group [9]. |
Amines/Amino group: Compounds and functional groups that contain a nitrogen atom with a lone pair of valence electrons [8]. Amino groups are represented by: −NH2
Amino acids: Molecules that contain an amino group and a carboxylic acid/carboxyl group. Osone: Molecules containing two alpha carbonyl groups [10]. Examples are Deoxyosones and deoxyglucosone. Where deoxy means containing less oxygen in the molecule than the compound from which it is derived [11]. Anomers: α- and β- forms of molecules [12] Carbohydrates, when in cyclic form, can exist in two isomer forms, α- and β- based on the position of the substituent at the anomeric center. For this isomers variation at the anomeric center, they are known as "anomers". The relative positions of the -CH2OH group and -OH (or -OR) group at the anomeric center distinguishes the α- from β- form. • α- form: The exocyclic O group at the anomeric center is on the opposite face to the -CH2OH group. • β- form: The exocyclic O group at the anomeric center is on the same face as the -CH2OH group. • A mixture of both α- and β- anomers: This is often represented using a "wavy" line to represent the bond. |
Caramelization
|
Caramelization is a specific type of pyrolysis reaction that occurs between sugar molecules when temperatures are above 105 °C (221 °F). This forms aromatic compounds
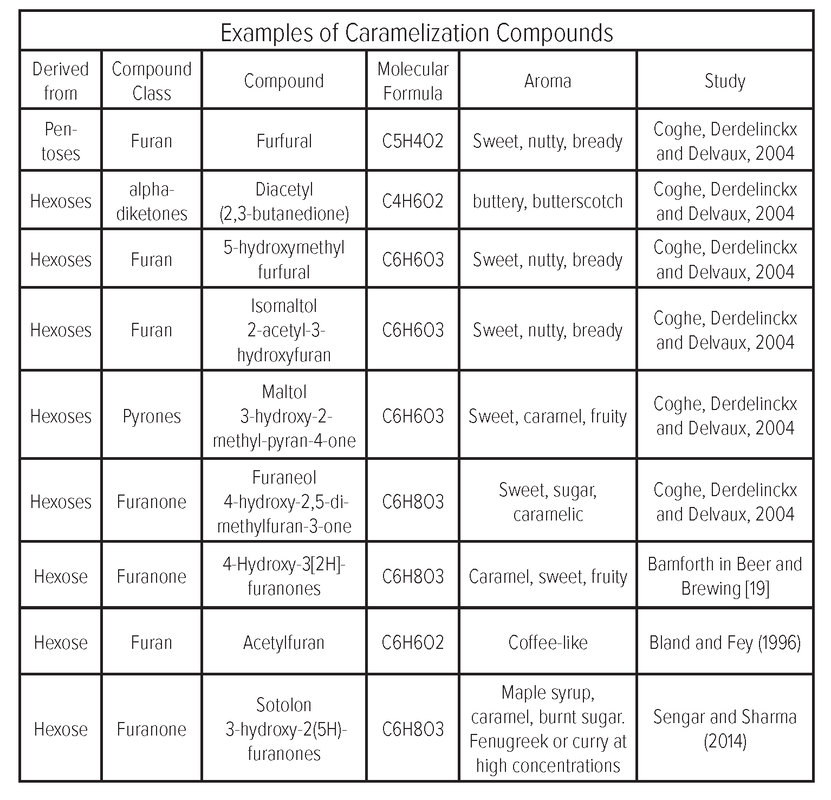
and non-aromatic colored compounds which have antioxidant potential due to their high molecular weight [14]. To summarize the well-explained caramelization reaction as noted by Corzo-Martínez, Corzo, Villamiel, & Del Castillo, (2012). Mechanism Precursors: Monosaccharides (Sugars) Process Lobry de BruynAlberda van Ekenstein transformation In a process that increases with increasing pH (more basic), an acid-base mechanism catalyzes the opening of a sugar’s hemiacetal ring structure and then its enolization. In this enolization process, an enol (a molecule containing a carbonyl group) has the carbonyl group transformed into a hydroxyl group thereby forming the molecule’s enol tautomer. After enolization, the formation of specific caramelization compounds is dependent on the acidity of the reaction environment. In acidic environments • Low amounts of isomeric carbohydrates are formed. An isomeric carbohydrate, is when the molecular formula of sugar is the same as another but the structure is different (isomer). An example of the isomers of C6H12O6 is glucose, fructose, and galactose. • Dehydration (removal of H2O) is favored as unbuffered acids act as catalysts, leading to furaldehyde compounds where: 2-furaldehyde forms from pentoses like arabinose, ribose, and xylose. 5-(hydroxymethyl)-2-furaldehyde (HMF) forms from hexoses (like glucose, fructose, annose, and galactose). In particular, higher yields of HMF are produced from fructose than from glucose. However, the enolization of glucose can be greatly increased by creating a buffered acidic solution like when a combination of phosphoric acid and pyridine is used as the catalyst compared to when phosphoric acid is used alone. |
In alkaline environment
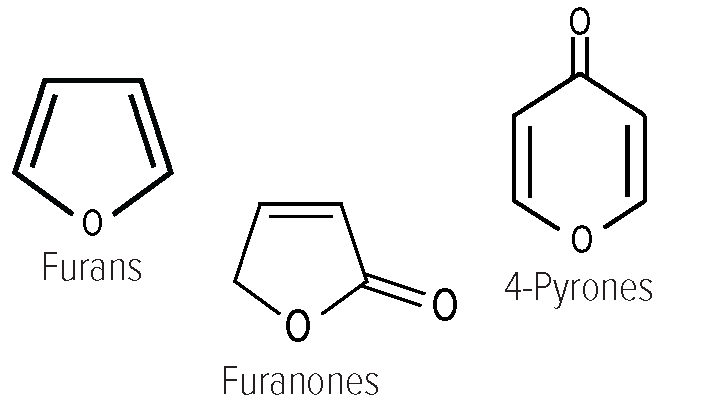
• Dehydration reactions occur more slowly than in neutral or acidic environments. • Fragmentation products caused by the degradation of larger molecules into smaller ones like acetol, acetoin, and diacetyl are detected. • In the presence of oxygen, oxidative fission forms organic acids including formic, and acetic acid. Process factors pH as the reaction rate is lowest at pH 7 and accelerated under both acidic (especially pH below 3) and basic (especially pH above 9) conditions. Temperature Temperatures as it occurs over 120°C but depends on the type of sugar [15]. • Fructose 105-110 °C (221 °F) • Glucose 150-160 °C (302 °F) • Galactose 160 °C (320 °F) • Sucrose 170 °C (338 °F) • Maltose 180 °C (360 °F) • Sugar type: When derived from glucose, caramel compounds mainly consist of colorless intermediates like reductones and dehydroreductones produced in the earlier stages of caramelization. Aroma Compounds [16] Given caramelization is a sugar-sugar reaction, the reaction products do not contain nitrogen. Non-aromatic browning products 90-95% of the products resulting from caramelization are non-volatile [17]. The major compounds contributing to color are • Caramelans (C24H36O18) • Caramelens (C36H50O25) • Caramelins (C125H188O80) Volatile aroma compound classes Carbocyclic compounds of: • Furans: Hydroxymethlyfurfural, Hydroxyacetylfrufuran • Furanones: Hydroxy dimethyl furanone, dihydroxy dimethyl furanone • 4-Pyrones: Malto from disaccharides and hydroxymalto from monosaccharides |
Maillard Reaction
First described by Maillard (1912) [23], Maillard reactions are non-pyrolytic reactions that occur between a sugar and an amino group, typically at temperatures above 50 °C [4]. However these reactions can occur at lower temperatures as is the case of dihydroxyacetone (DHA), which is the active ingredient in sunless tanner [24]. Yes, that means sunless tanner works by Maillard reactions on human skin.
|
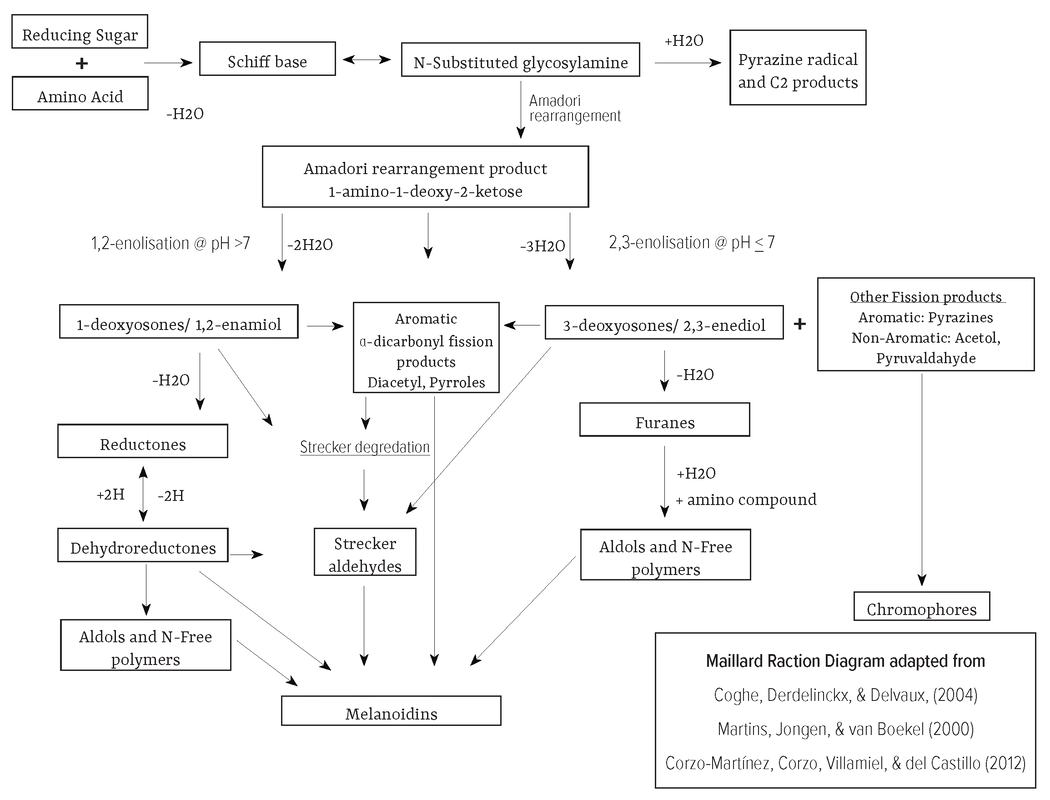
Mechanisms
First proposed by Hodge (1953) [25], the general Maillard reaction process is understood. However, the research on specific compound transformation is ongoing as the final products are influenced by the type and concentration of reactant, and the reaction conditions. We aggregated the descriptions of the reaction mechanisms by Coghe, Derdelinckx, & Delvaux, (2004), Martins, Jongen, van Boekel (2000), and Corzo-Martínez, Corzo, Villamiel, del Castillo (2012) for the following summary. Precursors Both the type of sugars and amino acids and their molar ratios significantly influence the rate of the Maillard reaction. Additionally low molecular weight compounds tend to be more reactive than those of higher molecular weight due to steric hindrance (the congestion caused by the physical presence of the surrounding ligands, which may slow down or prevent reactions at the atom [26]. Aldos Sugars Aldose, a class of monosaccharide composed of a carbon backbone chain and a carbonyl group on the endmost carbon atom [27]. Examples of Aldoses include: Mannose and Glucose. |
• Aldopentoses (5-carbon aldos) are usually more reactive than aldohexoses (6-carbon aldos).
• Monosaccharides are more reactive than disaccharides or oligosaccharides. • Aldoses appear to be more reactive than ketoses (has keytone group), probably due to the more sterically hindered carbonyl group of ketoses. • Generally, formation of browning compounds are maximized when sugar is in excess. Amino Group containing molecules Amino group containing molecules like amines, peptides, and proteins. In particular: • The most reactive amino acids are lysine or terminal amino acids and molecules with terminal amino acids like peptides (short chains of amino acids), and proteins. • Nonpolar and acidic amino acids had the lowest reactivity. • The more amino groups a molecule has, the more reactive the amino acid, and the more color that is created. • Excess amino acids, in basic conditions (higher pH values), maximized color formation (Renn and Sathe 1997). |
Initial Formation
|
Condensation of Reducing Sugar and Amino Acid
Spontaneous but promoted by high temperatures, nucleophilic amino groups in amino acids react with carbonyl groups in the reducing sugar [28]. This electron exchange between the amino group and reducing sugar creates a condensation reaction. Formation of Schiff base The elimination of water from the condensation reaction molecule forms a Schiff base. Formation of N-substituted aldosylamine (sometimes called glycosylamine) N-substituted aldosylamine is unstable because nitrogen is double bonded to a carbon thereby being electronegative. This causes the hydroxyl group to rearrange into a carbonyl group thereby isomerizing the molecule. The N-substituted aldosylamine can then either form an Adamori compound or be cleaved where: • Formation of Amadori compounds by Amadori rearrangement N-substituted aldosylamine transforms into an α-ketoamine (a compound that is both a ketone and an amine) and more specifically a 1-amino-1-deoxyketoses type molecule. This mechanism of formation, known as Amadori rearrangement, produces Amadori rearrangement products (ARP) which are also known as Amadori compounds. The Amadori compounds are precursors to other compounds that provide roasting’s characteristic flavors, aromas, and brown polymers. Villamiel, del Castro, and Corzo (2006) note that Amadori compounds are formed before the occurrence of sensory changes. • Formation of Flavor-active pyrazine radical and C2 products Have its sugar moiety cleaved before Amadori rearrangement to form flavor-active pyrazine radical and C2 products [29]. Aroma compound formation [3] The diversity of Maillard products are created by subsequent reactions that are environmentally influenced. These subsequent reactions can entail Amadori compounds undergoing various enolization reactions that result in different α-dicarbonyl compounds like deoxyosones (also known as deoxyglucosones). These α-dicarbonyl compounds are named due to their two vicinal (adjacent/neighboring) carbonyl groups. The specific type is influenced by the pH of the system and if the reaction is caused by condensation or carbohydrate fragmentation. pH ≤7 Formation of 3-deoxyosones (2,3-enediol) A pH of ≤7 promotes Amadori compounds to undergo 1,2-enolisations to the α-dicarbonyl compound 3-deoxyosones (2,3-enediol) due to a chemical equilibrium that favors the elimination of a proton and an amine [4]. Formation of furfural derivatives Furfural derivatives are formed from 3-deoxyosones that undergo dehydration and cyclization. The furfural derivative that forms is dependent on the sugar from which it was derived. For example: • Pentoses (5-carbon sugars) are transformed into furfural (caramel aroma). • Hexoses (6 carbon sugars) are transformed into hydroxymethylfurfural (HMF) (caramel aroma). • The addition of an amino compound can form melanoidin. |
pH >7
Formation of 1-deoxyosones (1,2-enamiol) Promotes Amadori compound degradation through 2,3 enolisation, leading to the formation of the α-dicarbonyl compound 1-deoxyosones (aka 1-deoxyglucosone/1,2-enamiol). This leads to either: • Strecker degradation (See section on Strecker Degradation) • Formation of Reductone ethers by the dehydration of 1-deoxyosones then cyclisation of the resulting compound. These reaction products which also exert antioxidative activity include: • Maltol (2-methyl-3-hydroxyγ-pyrone): Burnt-like aroma • Isomaltol (3-hydroxy-2-acetylfuran): Burnt-like aroma • Furanone (2-Furanone): Burnt-like aroma • 5-methyl-4-hydroxy-3(2H)-furanone (MHF) [30]: Derived from pentose, MHF has a meaty/brothy flavor. In beer, MHF is always found but at levels below the flavor threshold (8.3 mg/L in water). • Dehydroreductones can form by an equilibrium reaction in which hydrogen is removed from the reductone. They can then: • Change back into reductones with the addition of hydrogen. • Transform into aldehydes. • Transform into aldols (motif consisting of a 3-hydroxy ketone or 3-hydroxyaldehyde) and nitrogen-free polymers. Example pathway at pH >7: Pentos is changed into its Amadori compound. Thenits changed into its 1-deoxyosone product, 1-deoxydiketose, a C5 (meaning 5 carbon). 1-deoxydiketose then undergoes cyclization/dehydration/reduction to form MHF. Fission products from retro-aldol type reactions Favored in higher pH environments, carbonyl compounds and intact sugars can decompose into an aldehyde or ketone, plus another carbonyl compound [31]. Martins, Jongen, and van Boekel (2001) note that the resulting aldehydes and ketones include short-chain (C2-C4) α-dicarbonyl and α-hydroxycarbonyl compounds, hydroxyacetone derivatives, glyceraldehyde, and diketones. These resulting compounds are more reactive towards browning than the usual reducing sugars and even to such active intermediate products of aminocarbonyl reaction as the Amadori product and osones [32]. Diacetyl Can form by • Isomerization product of 1-deoxyglucosone. • 3-deoxyglucosone reaction with aniline or cyclohexylamine which also generates 2,3-pentanedione. Aroma: Butter popcorn Pyrroles Formed by α-dicarbonyl reacting with Proline (an amino acid) which creates 2-acetyl-1-pyrroline (sweet, nutty aroma notes). Pyrazine [3] •Formed by two aminoketone molecules condensing. •Roasted aromas are found in a multitude of foods, making pyrazines particularly important in food chemistry. |
Strecker Aldehydes from Strecker Degradation
|
Strecker aldehydes are significant aroma compounds due to their common occurrence in alcoholic beverages and low perception thresholds. They result from α-dicarbonyl’s (a molecule with 2 carbonyl groups) reaction with an amino group. While these α-dicarbonyls can result from Maillard reactions, they can also result from other formation pathways.
Precursors [4] • The amino acids that are most reactive with with α-dicarbonyl compounds are the three basic amino acids (those with side chains which contain nitrogen and resemble ammonia, as amonia is a base) [36] arginine, lysine, and histidine. Hydroxy amino acids serine and threonine are also highly reactive. • α-dicarbonyl compounds like glyoxal, methylglyoxal, diacetyl, or 3-deoxyosone. For a good article on this reaction mechanism in beer: Bravo, A., Herrera, J.C., Scherer, E., Ju-Nam, Y., Rübsam, H., Madrid, J., Zufall, C. and Rangel-Aldao, R., 2008. Formation of α-dicarbonyl compounds in beer during storage of pilsner. Journal of Agricultural and Food Chemistry, 56(11), pp.4134-4144. Retrieved from: www.academia.edu/83916101/Formation_of_%CE%B1_Dicarbonyl_Compounds_in_Beer_during_Storage_of_Pilsner • Carbonyl compound formed by the degradation of amino acids and proteins. • Lipid-derived carbonyls like α-keto acids [37], as their structure is similar to carbohydrate-derived carbonyls, are capable of causing Strecker-type aldehydes with amino acids [38]. These carbonyls can occur in a multitude of pathways which involve electronic rearrangement of an imine, which is triggered by the exit of the carboxylic proton under the acid conditions at which the reaction is produced [39]. Gernat, Brouwer, and Ottens, (2020) provides the example of oxidized lipids forming during malting of barley. For an insightful literature review on the topic: Hidalgo, F. J., & Zamora, R. (2016). Amino acid degradations are produced by lipid oxidation products. Critical Reviews in Food Science and Nutrition, 56(8), 1242–1252. Retrieved from: https://digital.csic.es/bitstream/10261/147635/1/Postprint_2016_CRFSNV56_P1242.pdf • Quinones from polyphenol oxidation, in the presence of two hydroxyl groups in ortho or para positions, were found by Delgado et al. (2015) to be capable of producing Strecker degradation of amino acids and amines in the absence of added oxidants. The study also found that the presence of two hydroxyl groups in the meta position and an additional aromatic ring in the polyphenol can inhibit the Strecker-degradation ability of the hydroxyl groups in ortho or para positions [40]. Reaction [4] • The carbonyl group of a dicarbonyl compound reacts with the amino group of an amino acid to form an unstable hemiaminal. |
• An imine is formed by the removal of water from the hemiaminal.
• The imine is then decarboxylated, through electron rearrangement to form an imine zwitterion. • If the second imine is dehydrated (removal of H20) it forms a Strecker aldehyde. • If water (H2O) is added, then it forms an amino alcohol. This, in turn, degrades into an α-ketoamine and a Strecker aldehyde. • Stryker aldehydes can undergo further reactions with amino acids to form colored compounds like melanoidins. Influenced by [3] • Higher concentrations of free amino acids and more drastic reaction conditions like higher temperatures or pressures result in higher Stricker aldehyde formation. • pH, as the formation of aldehydes shows a linear pH dependence. • In brewing, α-dicarbonyl compounds and amino acids react during wort boiling, especially at the beginning of the process, and then the concentration stabilizes due to a balance between formation and evaporation [41]. Types of Strecker aldehydes [42] • Methional (potato-like aroma) from methionine. • Methylpropanal (malty) from valine. • 2-methylbutanal (malty) from isoleucine. • 3-methylbutanal (malty) from leucine. • Phenylacetaldehyde (honey) from phenylalanine. • 2-acetyl-1-pyrroline (sweet popcorn aroma). Formation of Color Compounds Chromophores and Melanoidins As described by Coghe, Derdelinckx, & Delvaux (2004), Maillard reaction products can act as precursors. When condensed with each other and other molecules, they create colored compounds. This color formation is a useful analytical tool in approximating Maillard compound formation, and is used for the production of malted barley and coffee roasting. However, color is not directly correlated with the Maillard reaction products. For this reason, a roaster will use other sensory cues and, while this color can be analytically measured, the person doing the roasting is often responsible for visual inspection as technical color analysis takes too long to be useful [43]. As, the type of amino acid involved in Maillard reaction influences the amount of browning, they are classified into the following: • Most intense browning: Lysine, glycine, tryptophan, and tyrosine • Intermediate browning: Alanine, valine, leucine, isoleucine, phenylalanine, proline, methionine, asparagine, and glutamine • Low browning: The seven remaining amino acids, including two basic amino acids (histidine and arginine), the two acidic amino acids (aspartic acid and glutamic acid), the two hydroxy amino acids (serine and threonine) and the thiol-containing amino acid cysteine. |
Chromophores
|
While responsible for a significant part of the total color, only a limited number of chromophores produced by
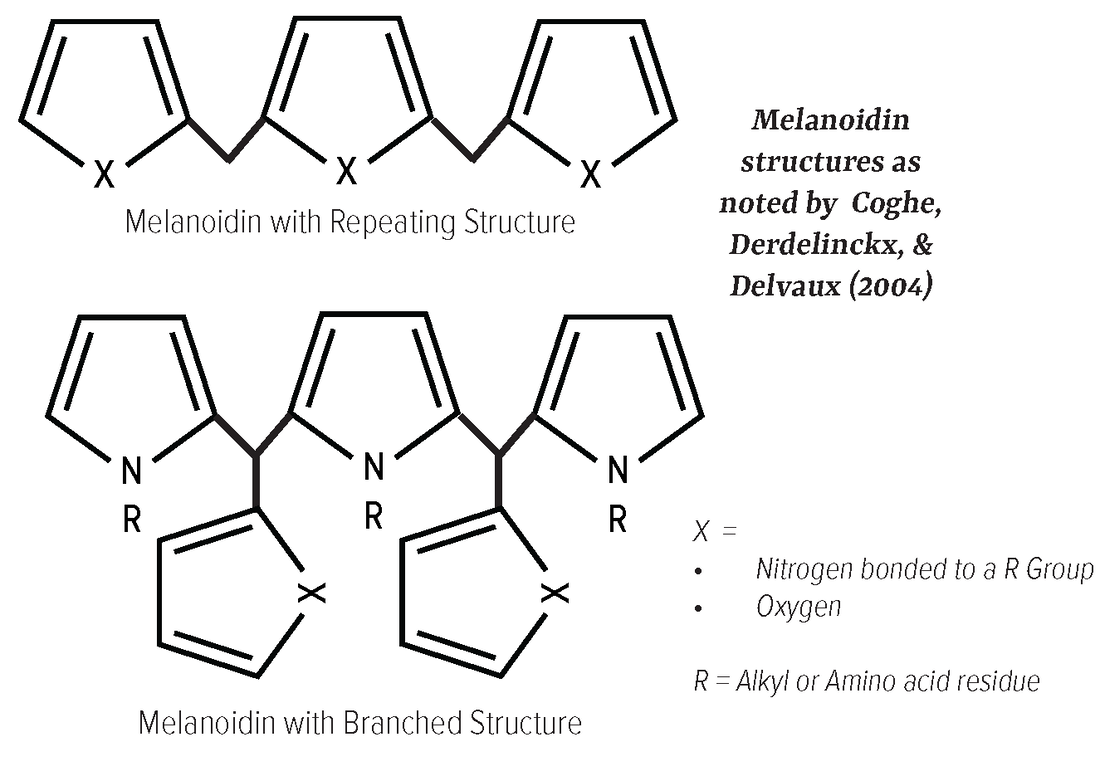
Maillard reaction are relevant for color production. Structure The currently identified Maillard chromophores are mostly composed of low molecular weight, two or three ring structures, that often contain oxygen or nitrogen, and are frequently linked by a –CH= group. Precursors First proposed by McWeeny et al. (1974) [44], significant precursors include: • Molecular classes of 3-deoxyosuloses and 3,4-dideoxyosulos- 3-enes, which in the case of glucose are 3-deoxyhexosulose (DH) and 3,4-dideoxyhexosuloses-3-ene (DDH) [2]. • Other major browning precursors, first proposed by Hoffman (1999) [45], include carbohydrate degradation products like deoxyosones, glyoxal, methylglyoxal, hydroxy-2-propanone, 3-hydroxy-2-butanon and glycoaldehyde [2]. Reaction A condensation reaction between methylene-active intermediates (molecules that have hydrogen atoms adjacent to 2 carbonyl groups [46]) and carbonyl compounds. This occurs because active methylene has hydrogen atoms that are especially reactive. Melanoidins Melanoidins are a class of polymers and copolymers that constitute the majority of the colored Maillard reaction products. Structure While their formation and constitution are difficult to study due to their disordered structures, melanoidins are proposed to be: • High molecular weight (up to 100,000 Da) • Composed of furan rings which can be repeating or branched. • Have nitrogen-containing polymers that also may contain a carbonyl, carboxyl, amine, amide, pyrrole, indole, azomethine, ester, anhydride, ether, methyl, and/or hydroxyl groups [2]. Precursors Colored intermediates and other reactive precursors including enaminol products, low-molecular-weight sugar analogues, and unsaturated carbonyl products [2]. |
Formation
While the research on melanoidin formation is ongoing, the general formation is a condensation reaction between Maillard reaction products and other precursors which is accelerated by an amine catalyst [2]. These formation methods, according to Coghe, Derdelinckx, and Delvaux (2004), include: • High molecular weight colored melanoidins can form from precursors of Maillard reaction products like furfural and low molecular weight chromophores that cross link with protiens. This occurs by cross-linking the high molecular weight protien's amino grops of lyseine or arginine via dehydration due to their nitrogen content. • Polymerisation of sugar degradation products, like dicarbonyl compounds or amino components [47], can form a branched structure where: • Early-stage Maillard compounds like 3-deoxyosuloses are polymerized via Aldol type condensation to form a basic melanoidin skeleton. • The amino acid’s carbohydrate side chains consisting of saccharides with intact glycosidic bonds may be attached to the melanoidin skeleton. Coloration in beer • Barley contains very low concentrations of naturally occurring pigmented substances, therefore most color is derived from roasting. • It has been observed that melanoidins from roasted malt are of considerably higher molecular weight than melanoidins of other malt types. Anti-oxidative activity of Maillard reaction products Maillard reaction products, and especially reductones and melanoidins, have a significant but not well understood antioxidant capability. This antioxidant effect has been associated with malting and brewing science. The term term “reductone” refers to beer’s increased anti-oxidative properties, which are produced by Maillard reaction products, formed via the 2,3-enediol pathway during malt kilning or roasting. To summarize Coghe, Derdelinckx, and Delvaux (2004) insight on the antioxidant activity of Maillard Reaction Products: • High molecular weight melanoidins possess a considerable antioxidative capability and are frequently termed “fast reducing agents”. However, Maillard antioxidants can be of both low and high molecular weight. • Maillard reaction produced volatile flavor-active heterocyclic compounds may possess antioxidative activity. This antioxidative activity for heterocyclic compounds may be attributed to its ring structure and is influenced by the ring’s degree of unsaturation as well as substituent type. Antioxidative reductone ethers which are flavor active include γ-pyrones like maltol and 5-hydroxymaltol. • Sulfur-containing heterocyclic compounds like pyrroles, γ-pyrones, furans, thiazoles, oxazoles, and furanones also possessed antioxidative activity. |
|
Antioxidant activity is influenced by
• Polarity of the reaction mixture as Maillard reaction products appeared to be highly antioxidative in hydrophilic but less effective in lipophilic solutions. • Color formation, as the development of antioxidative activity by Maillard reactions has been linearly correlated in model systems but not linear over a broad color range. This correlation may be dependent on the degree of heating and the type of method used to determine antioxidative activity. Charring, however, can cause antioxidant potential to drop significantly. • In coffee, melanoidin and phenol antiradical activity decreases as roasting intensity increases due to thermal degradation of Chlorogenic acids and pyrolysis of coffee components. However, the ability to prevent linoleic acid peroxidation was higher in melanoidins from dark roasted samples. • Dark specialty malt's antioxidative activity generally increases with malt color until 400 EBC units. However, the reducing power per unit of color was found to be the highest in pale malts. Above this limit, increases in total antioxidant activity were not observed because the compounds causing color may not be fully responsible for the reducing power. Therefore, the alteration of process temperatures, times, or rate of temperature rise may allow for a separate of color development and antioxidative activity. Maillard Reaction Process Factors [3] Coghe, Derdelinckx, & Delvaux (2004) note that the Maillard reaction is notoriously difficult to control, and the most important factors influencing the rate of reaction are: Time-temperature profile • Temperatures above 50 °C as higher temperatures increase reaction speed due to the increased kinetic energy of precursors. • Temperature increases shift reactions toward advanced Maillard reaction products due to the higher activation energies required for these reactions. • The reaction rate can be more than 10% higher for each 1°C increase in the range from 60°C to 100°C. Moisture content Water influences the viscosity which in turn increases the mobility of reactants. It also influences dissolution (acts as a solvent) and concentration (or dilution) of the reactants. It has been found that: • Maximal browning is generally accepted to occur at 0.65-0.70 aw. • In an intermediate moisture range, the reaction rate increases exponentially. • Excessive moisture, however, reduces the concentration of Maillard pre-cursors thereby reducing the ration rate. • The water produced via dehydration of sugars during Maillard reactions can increase the moisture level and lead to a further decreased rate of reaction. |
pH
An ideal pH range of 4–7. The initial pH of the product and the buffering capacity of the system influence the rate, direction and the profile of reaction and its products. As: • At low pH values (more basic), amino compounds are protonated and lack an unshared electron pair. Threfore, these are less reactive with carbonyl compounds in the initial stage of the Maillard reaction. • The decrease in reaction rate may be due to a deficiency of H+ ions, as these are required to catalyze the Amadori rearrangements. • The enolisation of Amadori compounds, the cleavage of intact sugars or deoxyosones and the formation of Strecker aldehydes are also influenced by the pH value. • The rate of browning is low at acidic pH values and increases with increasing pH to a maximum of pH 10 (Renn and Sathe 1997) [48]. Reaction Inihibitors • Sulfur compounds It has been observed that Sulphur dioxide, sulphites, and thiols inhibit Maillard reactions, however, the mechanisms are not well understood. It is believed the sulfur compounds react with reducing sugars and carbonyl products of the Maillard reaction to produce sulfonic acid derivatives, which have a diminished tendency to brown. • Sodium chloride concentration High concentrations of sodium chloride retarded the reaction rate of glucose with amino acids but did not change the browning rate of glucose with peptides [1]. Sources and Suggested Reading 1. Corzo-Martínez, M., Corzo, N., Villamiel, M., & Del Castillo, M. D. (2012). Browning reactions. Food biochemistry and food processing, 56-83. Retrieved from: www.academia.edu/33958572/Browning_Reactions 2. Martins, S., Jongen, W. M. F., van Boekel, M. (2000). A review of Maillard reaction in food and implications to kinetic modeling, Trends Food Sci. Tech. 11, 364-373. https://ucanr.edu/datastoreFiles/608-648.pdf 3. Coghe, S., Derdelinckx, G., & Delvaux, F. R. (2004). Effect of nonenzymatic browning on flavour, colour and antioxidative activity of dark specialty malts—a review. Monatsschr. Brauwiss, 57, 25-38. Retrieved from: http://themodernbrewhouse.com/wpcontent/uploads/2017/04/Coghe0604.pdf 4. Gernat, D.C., Brouwer, E. & Ottens, M. (2020). Aldehydes as Wort Off-Flavours in Alcohol-Free Beers—Origin and Control. Food Bioprocess Technol 13, 195–216. https://doi.org/10.1007/s11947-019-02374-z 5. Villa, K. (n.d.). The oxford companion to beer definition of candi sugar. Craft Beer & Brewing. Retrieved January 17, 2023, from https://beerandbrewing.com/dictionary/ 5SreTKcjau/ |
Resources and Suggested Reading
6. Wikipedia contributors. (2022, October 11). Aldehyde. In Wikipedia, The Free Encyclopedia. Retrieved 11:49, December 27, 2022, from https://en.wikipedia.org/w/index.php?title=Aldehyde&oldid=1115371925
7. Brown, W. H. and March, . Jerry (2022, December 16). carboxylic acid. Encyclopedia Britannica. https://www.britannica.com/science/carboxylic-acid
8. Wikipedia contributors. (2022, December 18). Amine. In Wikipedia, The Free Encyclopedia. Retrieved 20:33, January 19, 2023, from https://en.wikipedia.org/w/index.php?title=Amine&oldid=1128117784
9. Wikipedia contributors. (2022, December 14). Imine. In Wikipedia, The Free Encyclopedia. Retrieved 03:16, December 30, 2022, from https://en.wikipedia.org/w/index.php?title=Imine&oldid=1127304880
10. Merriam-Webster. (n.d.). Osone. In Merriam-Webster.com dictionary. Retrieved January 19, 2023, from www.merriam-webster.com/dictionary/osone
11. Merriam-Webster. (n.d.). Deoxy. In Merriam-Webster.com dictionary. Retrieved January 19, 2023, from https://www.merriam-webster.com/dictionary/deoxy
12. Hunt, D. I. R. (n.d.). What do the α- and β- forms look like? Ch25: Alpha and beta forms. Retrieved January 17, 2023, from https://www.chem.ucalgary.ca/courses/351/Carey5th/Ch25/ch25-3-1.html
13. Wikipedia contributors. (2022, December 29). Pyrolysis. In Wikipedia, The Free Encyclopedia. Retrieved 07:15, January 9, 2023, from https://en.wikipedia.org/w/index.php?title=Pyrolysis&oldid=1130214705
14. Benjakul, S., Visessanguan, W., Phongkanpai, V., & Tanaka, M. (2004, June 9). Antioxidative activity of caramelisation products and their preventive effect on lipid oxidation in fish mince. Science Direct. Retrieved January 17, 2023, from https://www.sciencedirect.com/science/article/abs/pii/S0308814604002973
15. Bamforth, C. W. (n.d.). The oxford companion to beer definition of caramelization. Craft Beer & Brewing. Retrieved January 17, 2023, from https://beerandbrewing.com/dictionary/2L0OfzScD2/
16. Wikipedia contributors. (2022, December 30). Caramelization. In Wikipedia, The Free Encyclopedia. Retrieved 07:07, January 9, 2023, from https://en.wikipedia.org/w/index.php?title=Caramelization&oldid=1130549318
17. Defaye, J., Fernández, J. G., & Ratsimba, V. (2000). Les molecules de la caramelisation: structure et methodologies de detection et d'evaluation. Actualite Chimique, 8(11), 24-27. Retrieved from: https://new.societechimiquedefrance.fr/wp-content/uploads/2019/12/2000-nov-236-NT10-Defaye.pdf
18. Wikipedia contributors. (2023, January 10). 4-Pyrone. In Wikipedia, The Free Encyclopedia. Retrieved 09:52, January 17, 2023, from https://en.wikipedia.org/w/index.php?title=4-Pyrone&oldid=1132816115
19. Bamforth, C. W. (n.d.). Caramelization. Craft Beer & Brewing. Retrieved January 15, 2023, from https://beerandbrewing.com/dictionary/2L0OfzScD2/
20. Blank, I., & Fay, L. B. (1996). Formation of 4-hydroxy-2, 5-dimethyl-3 (2 H)-furanone and 4-hydroxy-2 (or 5)-ethyl-5 (or 2)-methyl-3 (2 H)-furanone through Maillard reaction based on pentose sugars. journal of Agricultural and Food Chemistry, 44(2), 531-536. Retrieved from: https://www.researchgate.net/publication/238389024_Formation_of_4Hydroxy25-dimethyl-32_H_-furanone_and_4Hydroxy2or_5-ethyl-5or_2-methyl-32_H_furanone_through_Maillard_Reaction_
Based_on_Pentose_Sugars
21. Sengar, G., & Sharma, H. K. (2014). Food caramels: a review. Journal of food science and technology, 51(9), 1686-1696. Retrieved from: https://pubchem.ncbi.nlm.nih.gov/compound/3-Hydroxy-4_5-dimethylfuran-2_5H_-one
22. Wikipedia contributors. (2022, December 12). Sotolon. In Wikipedia, The Free Encyclopedia. Retrieved 09:54, January 17, 2023, from https://en.wikipedia.org/w/index.php?title=Sotolon&oldid=1126926428
23. Maillard, L. C. (1912). Action des acides amines sur les sucres: formation des melanoidines par voie methodique. CR Acad Sci, 154, 66-68.
24. Wickett, R. R. (2005, May 30). How do sunless tanners work? Scientific American. Retrieved January 19, 2023, from www.scientificamerican.com/article/how-do-sunless-tanners-wo/
25. Hodge, J. E. (1953). Chemistry of browning reactions in model systems. J. Agric. Food Chem., 46, 2599-2600.
26. Gunawardena, G. (2022, February 28). Steric Hindrance. LibreTexts Chemistry . Retrieved January 17, 2023, from https://chem.libretexts.org/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Steric_Hindrance
27. Wikipedia contributors. (2022, May 17). Aldose. In Wikipedia, The Free Encyclopedia. Retrieved 11:31, December 27, 2022, from https://en.wikipedia.org/w/index.php?title=Aldose&oldid=1088410633
Wikipedia contributors. (2022, December 8). Reducing sugar. In Wikipedia, The Free Encyclopedia. Retrieved 11:43, December 27, 2022, from https://en.wikipedia.org/w/index.php?title=Reducing_sugar&oldid=1126279716
28. Hayashi, T., Mase, S. and Namiki, M., 1986. Formation of three-carbon sugar fragment at an early stage of the browning reaction of sugar with amines or amino acids. Agricultural and biological chemistry, 50(8), pp.1959-1964. Retrieved from: https://www.tandfonline.com/doi/epdf/10.1080/00021369.1986.10867691
7. Brown, W. H. and March, . Jerry (2022, December 16). carboxylic acid. Encyclopedia Britannica. https://www.britannica.com/science/carboxylic-acid
8. Wikipedia contributors. (2022, December 18). Amine. In Wikipedia, The Free Encyclopedia. Retrieved 20:33, January 19, 2023, from https://en.wikipedia.org/w/index.php?title=Amine&oldid=1128117784
9. Wikipedia contributors. (2022, December 14). Imine. In Wikipedia, The Free Encyclopedia. Retrieved 03:16, December 30, 2022, from https://en.wikipedia.org/w/index.php?title=Imine&oldid=1127304880
10. Merriam-Webster. (n.d.). Osone. In Merriam-Webster.com dictionary. Retrieved January 19, 2023, from www.merriam-webster.com/dictionary/osone
11. Merriam-Webster. (n.d.). Deoxy. In Merriam-Webster.com dictionary. Retrieved January 19, 2023, from https://www.merriam-webster.com/dictionary/deoxy
12. Hunt, D. I. R. (n.d.). What do the α- and β- forms look like? Ch25: Alpha and beta forms. Retrieved January 17, 2023, from https://www.chem.ucalgary.ca/courses/351/Carey5th/Ch25/ch25-3-1.html
13. Wikipedia contributors. (2022, December 29). Pyrolysis. In Wikipedia, The Free Encyclopedia. Retrieved 07:15, January 9, 2023, from https://en.wikipedia.org/w/index.php?title=Pyrolysis&oldid=1130214705
14. Benjakul, S., Visessanguan, W., Phongkanpai, V., & Tanaka, M. (2004, June 9). Antioxidative activity of caramelisation products and their preventive effect on lipid oxidation in fish mince. Science Direct. Retrieved January 17, 2023, from https://www.sciencedirect.com/science/article/abs/pii/S0308814604002973
15. Bamforth, C. W. (n.d.). The oxford companion to beer definition of caramelization. Craft Beer & Brewing. Retrieved January 17, 2023, from https://beerandbrewing.com/dictionary/2L0OfzScD2/
16. Wikipedia contributors. (2022, December 30). Caramelization. In Wikipedia, The Free Encyclopedia. Retrieved 07:07, January 9, 2023, from https://en.wikipedia.org/w/index.php?title=Caramelization&oldid=1130549318
17. Defaye, J., Fernández, J. G., & Ratsimba, V. (2000). Les molecules de la caramelisation: structure et methodologies de detection et d'evaluation. Actualite Chimique, 8(11), 24-27. Retrieved from: https://new.societechimiquedefrance.fr/wp-content/uploads/2019/12/2000-nov-236-NT10-Defaye.pdf
18. Wikipedia contributors. (2023, January 10). 4-Pyrone. In Wikipedia, The Free Encyclopedia. Retrieved 09:52, January 17, 2023, from https://en.wikipedia.org/w/index.php?title=4-Pyrone&oldid=1132816115
19. Bamforth, C. W. (n.d.). Caramelization. Craft Beer & Brewing. Retrieved January 15, 2023, from https://beerandbrewing.com/dictionary/2L0OfzScD2/
20. Blank, I., & Fay, L. B. (1996). Formation of 4-hydroxy-2, 5-dimethyl-3 (2 H)-furanone and 4-hydroxy-2 (or 5)-ethyl-5 (or 2)-methyl-3 (2 H)-furanone through Maillard reaction based on pentose sugars. journal of Agricultural and Food Chemistry, 44(2), 531-536. Retrieved from: https://www.researchgate.net/publication/238389024_Formation_of_4Hydroxy25-dimethyl-32_H_-furanone_and_4Hydroxy2or_5-ethyl-5or_2-methyl-32_H_furanone_through_Maillard_Reaction_
Based_on_Pentose_Sugars
21. Sengar, G., & Sharma, H. K. (2014). Food caramels: a review. Journal of food science and technology, 51(9), 1686-1696. Retrieved from: https://pubchem.ncbi.nlm.nih.gov/compound/3-Hydroxy-4_5-dimethylfuran-2_5H_-one
22. Wikipedia contributors. (2022, December 12). Sotolon. In Wikipedia, The Free Encyclopedia. Retrieved 09:54, January 17, 2023, from https://en.wikipedia.org/w/index.php?title=Sotolon&oldid=1126926428
23. Maillard, L. C. (1912). Action des acides amines sur les sucres: formation des melanoidines par voie methodique. CR Acad Sci, 154, 66-68.
24. Wickett, R. R. (2005, May 30). How do sunless tanners work? Scientific American. Retrieved January 19, 2023, from www.scientificamerican.com/article/how-do-sunless-tanners-wo/
25. Hodge, J. E. (1953). Chemistry of browning reactions in model systems. J. Agric. Food Chem., 46, 2599-2600.
26. Gunawardena, G. (2022, February 28). Steric Hindrance. LibreTexts Chemistry . Retrieved January 17, 2023, from https://chem.libretexts.org/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Steric_Hindrance
27. Wikipedia contributors. (2022, May 17). Aldose. In Wikipedia, The Free Encyclopedia. Retrieved 11:31, December 27, 2022, from https://en.wikipedia.org/w/index.php?title=Aldose&oldid=1088410633
Wikipedia contributors. (2022, December 8). Reducing sugar. In Wikipedia, The Free Encyclopedia. Retrieved 11:43, December 27, 2022, from https://en.wikipedia.org/w/index.php?title=Reducing_sugar&oldid=1126279716
28. Hayashi, T., Mase, S. and Namiki, M., 1986. Formation of three-carbon sugar fragment at an early stage of the browning reaction of sugar with amines or amino acids. Agricultural and biological chemistry, 50(8), pp.1959-1964. Retrieved from: https://www.tandfonline.com/doi/epdf/10.1080/00021369.1986.10867691
Resources and Suggested Reading
29. Mackie, A. E., & Slaughter, J. C. (2002). Formation of 4-Hydroxyfuranones and their Precursors during Production of Worts and Beers. Journal of the Institute of Brewing, 108(3), 336-343. Retrieved from: https://onlinelibrary.wiley.com
/doi/pdf/10.1002/j.2050-0416.2002.tb00558.x
30. Illustrated Glossary of Organic Chemistry. UCLA Chemistry and Biochemistry . (n.d.). Retrieved January 17, 2023, from https://www.chem.ucla.edu/~harding/IGOC/R/retro_aldol_reaction.html
31. Hayashi, T., Namiki, M.: Role of sugar fragmentation in an early stage browning of amino-carbonyl reaction of sugar with amino-acid, Agr. Biol. Chem. 50, 1965-1970, 1986. https://www.tandfonline.com/doi/pdf/10.1080
/00021369.1986.10867692
32. Weenen, H.: Reactive intermediates and carbohydrate fragmentation in Maillard chemistry, Food Chem. 62, 393-401, 1998 www.academia.edu/57169678/Reactive_intermediates_and_carbohydrate_fragmentation_in_Maillard_chemistry
33. Wikipedia contributors. (2022, February 14). Hydroxyacetone. In Wikipedia, The Free Encyclopedia. Retrieved 10:31, January 17, 2023, from https://en.wikipedia.org/w/index.php?title=Hydroxyacetone&oldid=1071829987
34. Wikipedia contributors. (2022, October 29). Methylglyoxal. In Wikipedia, The Free Encyclopedia. Retrieved 10:34, January 17, 2023, from https://en.wikipedia.org/w/index.php?title=Methylglyoxal&oldid=1118889521
35. University of Wisconsin Department of Chemistry. (n.d.). Acidic and Basic Amino Acids. ChemPages Netorials. Retrieved January 19, 2023, from https://www2.chem.wisc.edu/deptfiles/genchem/netorial/modules/biomolecules
/modules/protein1/prot14.htm
36. Hidalgo, F. J., Delgado, R. M., & Zamora, R. (2013). Intermediate role of α-keto acids in the formation of Strecker aldehydes. Food chemistry, 141(2), 1140-1146. https://doi.org/10.1016/j.foodchem.2013.03.068
37. Hidalgo, F. J., & Zamora, R. (2004). Strecker-type degradation produced by the lipid oxidation products 4, 5-epoxy-2-alkenals. Journal of Agricultural and Food Chemistry, 52(23), 7126-7131. https://doi.org/10.1021/jf048883r.
38. For an insightful literature review on the topic: Hidalgo, F. J., & Zamora, R. (2016). Amino acid degradations are produced by lipid oxidation products. Critical Reviews in Food Science and Nutrition, 56(8), 1242–1252. https://doi.org/10.1080/10408398.2012.761173 Retrieved from https://digital.csic.es/bitstream/10261/147635
/1/Postprint_2016_CRFSNV56_P1242.pdf
39. Delgado, R. M., Zamora, R., & Hidalgo, F. J. (2015). Contribution of phenolic compounds to food flavors: Strecker-type degradation of amines and amino acids produced by o-and p-diphenols. Journal of agricultural and food chemistry, 63(1), 312-318. https://doi.org/10.1021/jf5047317.
40. Piornos, J. A., Koussissi, E., Balagiannis, D. P., Brouwer, E., & Parker, J. K. (2022). Alcohol-free and low-alcohol beers: Aroma chemistry and sensory characteristics. Comprehensive Reviews in Food Science and Food Safety. https://doi.org/10.1111/1541-4337.13068
41. Piornos, J.A., Delgado, A., de La Burgade, R.C., Methven, L., Balagiannis, D.P., Koussissi, E., Brouwer, E. and Parker, J.K., 2019. Orthonasal and retronasal detection thresholds of 26 aroma compounds in a model alcohol-free beer: Effect of threshold calculation method. Food Research International, 123, pp.317-326. Retrieved from: https://centaur.reading.ac.uk/83299/5/08%20Apr%20Manuscript%20Reviewed%20for%20Centaur.pdf
42. McWeeny, D.J., Knowels, M.E. and Hearne, J.F. (1974) ‘The Chemistry of Non-enzymic Browning and Its Control by Sulphites’ in J. Sci. Food Agric. 25, 735–746. https://doi.org/10.1002/jsfa.2740250616
43. Hofmann, T. (1999). Quantitative studies on the role of browning precursors in the Maillard reaction of pentoses and hexoses with L-alanine. European food research and technology, 209(2), 113-121. https://link.springer.com/article/10.1007/s002170050468
44. Hunt, D. I. R. (n.d.). Active Methylenes. University of Calgary. Retrieved January 17, 2023, from https://www.chem.ucalgary.ca/courses/350/Carey5th/Ch21/ch21-1-2.html
45. Cämmerer, B., & Kroh, L. W. (1995). Investigation of the influence of reaction conditions on the elementary composition of melanoidins. Food Chemistry, 53(1), 55-59. https://doi.org/10.1016/0308-8146(95)95786-6
46. Renn, P. T., & Sathe, S. K. (1997). Effects of pH, temperature, and reactant molar ratio on L-leucine and D-glucose Maillard browning reaction in an aqueous system. Journal of Agricultural and Food Chemistry, 45(10), 3782-3787
47. Gunawardena, G. (2022, February 28). Steric Hindrance. LibreTexts Chemistry . Retrieved January 17, 2023, from https://chem.libretexts.org/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Steric_Hindrance
PUBLISHED BY: HAWAI'I BEVERAGE GUIDE
MENU
|
HOME
|
SUBSCRIBE |
DIGITAL
|
BEVERAGE
|
NEws and
|
ABOUT |
CONTACT |
©2022 Hawaii Beverage Guide
Terms & Conditions
Terms & Conditions
- Subscribe
- Digital Edition
-
Beverage Guide
- Flavor and Cocktail Construction >
- Production Fundamentals >
- Non-Alcoholic Beverages >
-
Beer
>
- Cider >
- Sake
-
Spirits
>
-
Wine
>
- Kamaʻāina Wine Makers >
-
Winemaking
>
- A Guide to: High Sugar Residual Wines
- A Guide to: Post Fermentation Flavor Adjustments
- A Guide to: Post Fermentation Process: Stabilization
- A Guide to: Wine Prefermentation Practices
- A Guide to: Wine Microbes
- A Guide to: Wine Alcoholic Fermentation Physical Environment
- A Guide to: Wine Fermentation Chemical Environment
- A Guide to: Wine Bottling
- A Guide to: Wine Faults
- A Guide to: Wine Polyphenols
- A Guide to: Wine Aroma Compounds: Pt 1
- A Guide to: Wine Aroma Compounds: Pt 2
- A Guide to Viticulture
- Red and White Grape Aroma Compounds
- Wine Styles >
- Business Strategy
- News and Events
- About
- Production Fundamentals
- Flower Aroma Compounds
- Flavor Pairing and Recipe Development