Herb and Spice Aroma Compounds
By: Brent Nakano
Understanding the aromatic compounds in common cocktail ingredients can be helpful with cocktail development, as seen in the Pairing of Aroma Compounds segment in Guide to Recipe Development, and to provide a better understanding of flavor descriptors that can translate to other beverages. The following are the findings in the academic literature of aroma compounds that create each herb’s “signature” flavor. The list is meant to provide rough perspective on each listed herb’s aroma compounds as found by Gas Chromatography and Mass Spectrometry, though one should take into account that variations occur due to factors like if the herb was dried or not dried, and the extraction technique used, the specific cultivar analyzed, species, pests, soil conditions, harvest season, geographical location, climatic and growth conditions.
Fresh Herbs (Aromatic Component)
In refreshing cocktails, mint is the classic fresh herb. Its popularity is closely followed by basil, however, rosemary, thyme, and sage are also great alternatives. All fresh herbs can be muddled directly into the cocktail to add a pop of aromatic freshness. Another option is a herbal liqueur or the addition of essential oils. Jonas et al (2021) in a study of sage notes that while all monoterpenes remained nearly unchanged during drying, highly volatile compounds were decreased and almost a total loss occurred for 3-(methylthio)propanal, phenylacetaldehyde, and (Z)-3-hexenal. (Z)-3-hexenal is known as leaf aldehyde because it is common in most leaves of plants.
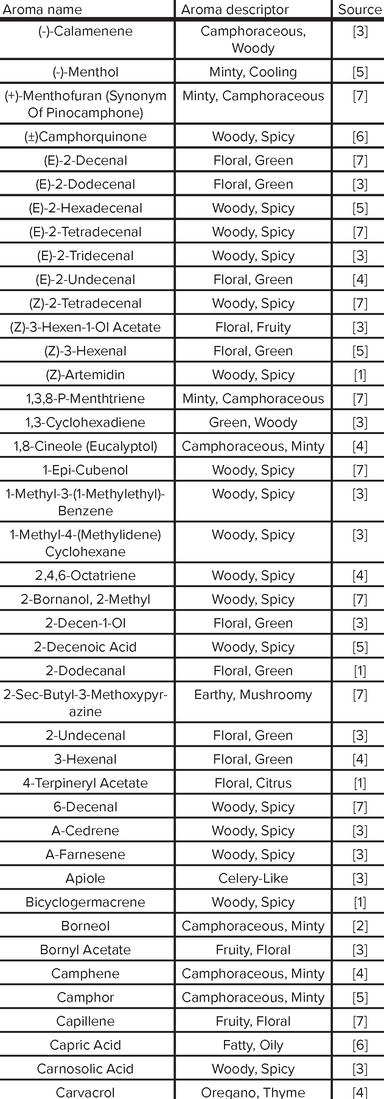
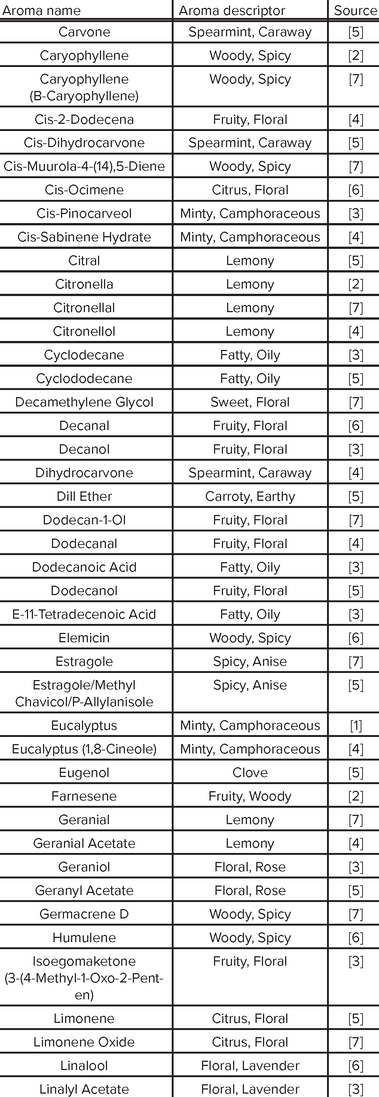
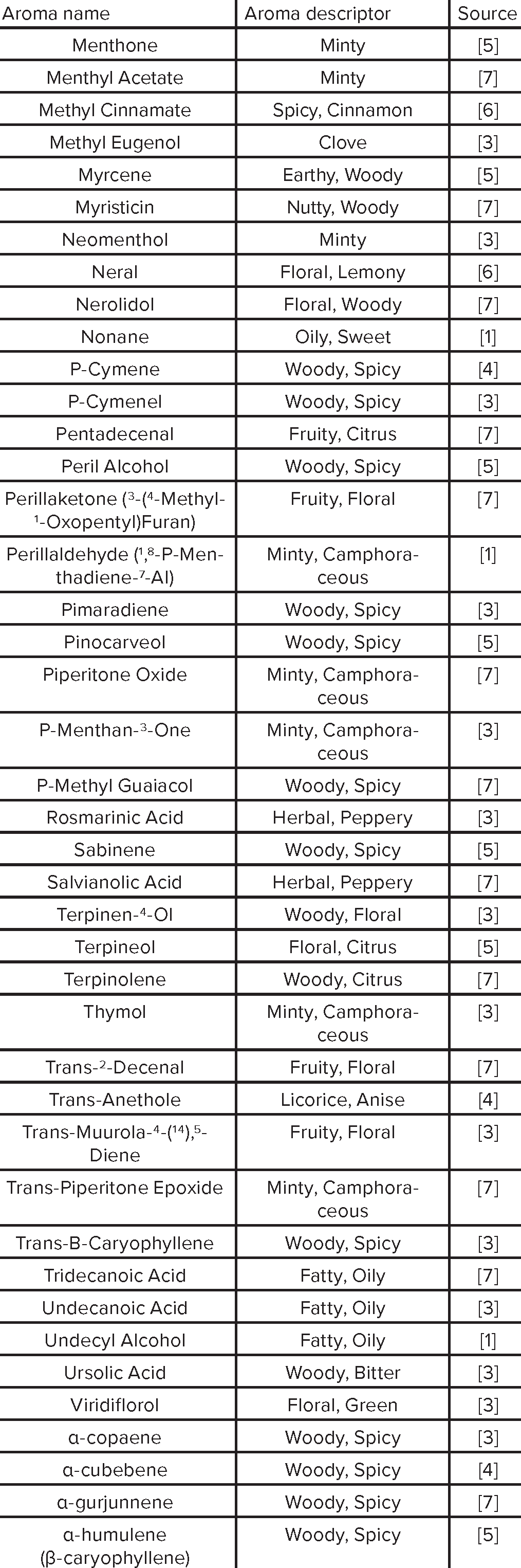
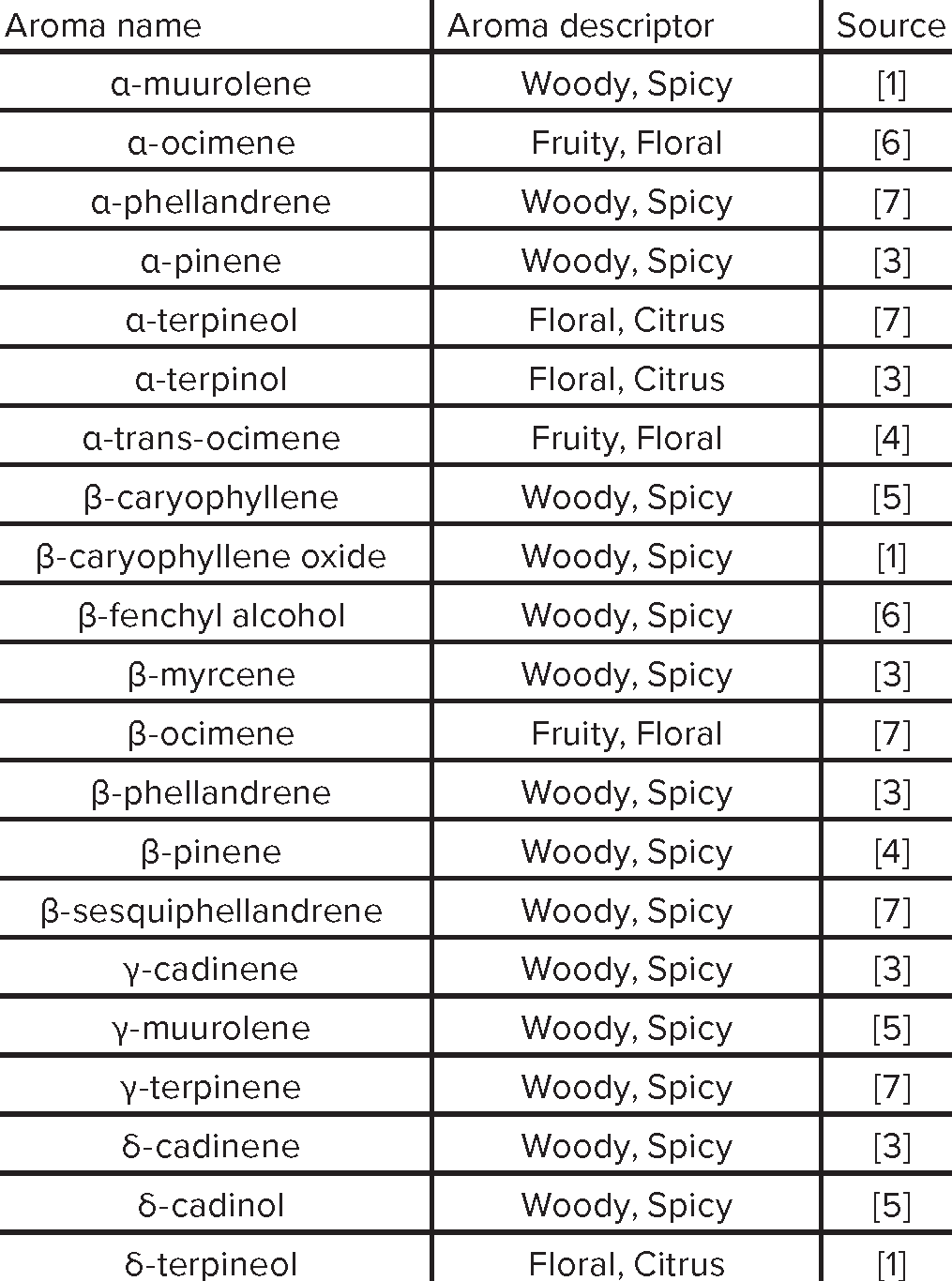
Aroma Compound Reference List
|
Source of Aroma Compound Descriptors
[1] Anupama, K. R., & Anju, K. (2019). Characterization of volatile compounds from essential oils of Ocimum gratissimum L. and Ocimum sanctum L. using GC–MS. Food Chemistry, 298, 124793. [2] Li, D., Liu, Y., Zhang, W., Sun, Z., & Li, J. (2019). Menthofuran and its stereoisomers in peppermint essential oil: Antioxidant and anti-inflammatory activities. Food Chemistry, 294, 139-147. [3] Kumar, A., & Kaur, S. (2019). Recent trends in the isolation, characterization and applications of volatile compounds from plants. Molecules, 24(22), 3638. [4] Zhang, W., Wang, X., Chen, J., Wang, Y., & Wang, J. (2019). Characterization of volatile compounds in spearmint (Mentha spicata L.) essential oil and their antimicrobial activities. Food Chemistry, 284, 268-274. [5] Wang, Y., Chen, J., Wang, X., Xu, J., & Wang, J. (2019). Isolation and characterization of volatile compounds from peppermint oil and their anti-inflammatory activities. Food Chemistry, 283, 306-314. [6]El-Hakim, N. A., & El-Demerdash, M. A. (2019). Chemical composition and biological activities of camphorquinone. Molecules, 24(1), 97. [7] Khan, A. A., & Khan, A. U. (2019). Aroma volatiles of calamintha nepeta (L.) herbal tea: characterization and identification by GC–MS. Food Chemistry, 273, 581-587. |
Basil
|
General Primary Aroma Compounds
Linalool, eugenol, estragole Sweet Basil and Genovese Basil (Ocimum basilicum) Primary Aroma Compounds Linalool, eugenol [2] Compounds present in lesser amounts •Calín-Sánchez et al (2012): Methyleugenol, eugenol, eucalyptol. •De Martino et al (2022) [2]: Eucalyptol (4.6–12.3%), thymol (0.2–8.4%), cis-muurola-4-(14),5-diene (0.8–11.6%), trans-muurola-4-(14),5-diene (1.3–8.8%) and 1-epi-cubenol (1.8–14.6%). •Lee et al. (2005) [3]: Estragole/Methyl chavicol (1-methoxy-4-(2-propenyl) benzene) (20.5–56.2%), eucalyptol/1,8-cineole) (2.9–4.37%), methyl cinnamate (12.9%), methyleugenol Thai basil (O. basilicum var. thyrsiflo rum) Aroma Compounds [4]: Estragole (methyl chavicol) (452.80 μg/mL), geranial (180.57 μg/mL), and neral (151.16 μg/mL) Lemon basil (O. citriodorum) Aroma Compounds [4]: Estragole (methylchavicol) (98.22 μg/mL), citral (9.55 μg/mL), and neral (6.32 μg/mL) |
White Holy basil (O. sanctum var. Rama) Aroma Compounds [4]: Methyl eugenol (98.44 μg/mL), α-cubebene (9.94 μg/mL), and α-copaene (4.74 μg/mL).
Red Holy basil (O. sanctum var. Shyama) Aroma Compounds [4]: Methyl eugenol (683.90 μg/mL), β-caryophyllene (145.81 μg/mL), and α-cubebene (104.70 μg/mL) Tree basil (O. gratissimum) Aroma Compounds [4]: Eugenol (408.00 μg/mL), α-ocimene (256.45 μg/mL), and γ-muurolene (91.57 μg/mL) Principal Component Analysis (PCA) Groupings of Basil sold in Thai markets [4] •Thai basil and Lemon basil: A distinctive anise, and citrus aroma from estragole, geranial, and neral. •Red and white Holy Basil: Spice-like aroma from methyl eugenol, β-caryophyllene, and α-cubebene. For more insight into the different variations of Basil: Simon, J. E., Morales, M. R., Phippen, W. B., Vieira, R. F., & Hao, Z. (1999). Basil: A source of aroma compounds and a popular culinary and ornamental herb. Perspectives on new crops and new uses, 16, 499-505. Retrieved from: http://www2.hawaii.edu/~theodore/Images/basil.pdf Du, P.; Yuan, H.; Chen, Y.; Zhou, H.; Zhang, Y.; Huang, M.; Jiangfang, Y.; Su, R.; Chen, Q.; Lai, J.; et al. Identification of Key Aromatic Compounds in Basil (Ocimum L.) Using Sensory Evaluation, Metabolomics, and Volatilomics Analysis. Metabolites 2023, 13, 85. https://doi.org/10.3390/metabo13010085 |
Bay leaf (Laurus nobilis)
|
Primary aroma compounds
1,8-cineole Other Significant aroma compounds: Sellami et al (2011) [5]: Linalool, trans-sabinene hydrate, α-terpinyl-acetate, methyl eugenol, sabinene, terpinen-4-ol and eugenol. Where the “spicy” aroma of bay leaves is from the eugenol, methyl eugenol and elemicin. |
Kilic et al (2004) [6]:
•α-pinene (3.9-5.0%), β-pinene(3.0-3.8%), sabinene(7.1-7.6%), α-terpinyl acetate(4.8-6.5%), α-terpineol(1.3-1.8%), linalool (trace-1.5%), eugenol (1.6-0.1%), β-elemene (1.4-1.8%), δ-terpinyl acetate (0.2-0.4%) •Killic et al (2004) also notes that harvest time influences the aromatic compounds in Bay Leaf as young shoots contained higher amounts of α-pinene, β-pinene, linalool, α-terpineol, 2-hydroxy-1,8-cineole, and some sesquiterpenes whereas older shoot leaves had 1,8-cineole, sabinene,sabinene hydrates, terpinene-4-ol, α-terpinyl acetate, eugenol, and eugenol methyl ether Other species of “Bay Leaf [7] (with different aroma compound compositions) California bay laurel (Umbellularia californica), Indian bay leaf (Cinnamomum tamala), Indonesian bay leaf (Syzygium polyanthum), West Indian bay laurel (Pimenta racemosa), and Mexican bay laurel (Litsea glaucescens) |
Coriander/Cilantro
|
Common cultivars [8]
While there are two different cultivars, the available research did not make a distinction between the aroma compounds of the cultivars. •Coriandrum sativum L. var. vulgare: Fruits 3–5 mm diameter with essential oil yields of 0.1%–0.35% (v/w) •Coriandrum sativum L. var. Microcarpum: Fruits 1.5–3 mm diameter with EO yields of 0.8%–1.8% (v/w) Coriander Globular-shaped seeds/fruits Primary Aroma compounds Linalool Other aroma compounds Łyczko et al (2021) [9]: Linalool (57.5–75.1%), geranyl acetate (8.9–24.5%), α-pinene (2.3–23.2%), terpineol (0.08–5.3%), geraniol (0.5–2.3%) and citronellol (0.6–1.6%) Shahwar et al (2012) [10]: Linalool (55.59%), γ-terpinene (7.47%), α-pinene (7.14%), camphor (5.59%), decanal (4.69%), geranyl acetate (4.24%), limonene (3.10%), geraniol (2.23%), camphene (1.78%), and D-limonene (1.36%) Mandal et al. (2015) [11]: Linalool (58.0–80.3%), γ-terpinene (0.3%–11.2%), α-pinene (0.2%–10.9%), p-cymene (0.1%–8.1%), camphor (3.0%–5.1%) and geranyl acetate (0.2%–5.4%) Bhuiyan et al (2009) [12]: Seed oil/Fruit Oil: Linalool (37.7%), geranyl acetate (17.6%) and γ-terpinene (14.4%), β-pinene (1.8%), m-cymene (1.3%), citronellal (2.0%), citronellol (1.3%), citral (1.4%), geraniol (1.9%), citronellyl acetate (1.4%), a-cedrene (3.9%), and a-farnesene (1.2%) and β-sesquiphellandrene (1.6%). |
Cilantro (the leaves)
Green leaf volatiles like (Z)-3-hexenol, (E)-2-hexenol, and (Z)-3-hexenyl acetate form after mechanical damage of plant cells due to enzymatic degradation of cell-wall lipids and have a characteristic odor referred to as the “green note” [13]. Primary aroma compounds In fresh leaves: (Z)-3-hexen-1-ol acetate, which then disappears in the creation of essential oils, probably due to degradation during the distillation process [13]. Other significant aroma compounds In fresh leaves Łyczko et al (2021) [9]: (Z)-3-hexen-1-ol acetate (34.54 ± 2.08%), (Z)-3-hexenal (17.38 ± 0.38%), nonane (9.28 ± 1.20%), limonene (2.43 ± 0.22%), linalool (1.44 ± 0.02%), decanol (4.76 ± 0.56%), dodecanal (1.76 ± 0.30%), , dodecanol (1.35 ± 0.67%) and (Z)-2-tetradecenal (4.77 ± 0.50%), Decanal (4.53 ± 0.13%), (E)-2-dodecenal (4.18 ± 0.36%), (E)-2-decenal. Kumar et al (2022) [13]: (Z)-3-hexenol, 1-decanol, (E)-2-dodecenal, nonane and (E)-2-tetradecenal In essential oil Shahwar et al (2012) [10]: (E)-2-decenal (32.23%), linalool (13.97%), (E)-2-dodecenal (7.51%), (E)-2-tetradecenal (6.56%), 2-decen-1-ol (5.45%), (E)-2-undecenal (4.31%), dodecanal (4.07%), (E)-2-tridecenal (3.00%), (E)-2-hexadecenal (2.94%), pentadecenal (2.47%), and α-pinene (1.9%). Mandal et al. (2015) [11]: Decanal (19.09%), trans-2-decenal (17.54%), 2-decen-1-ol (12.33%) and cyclodecane (12.15%), cis-2-dodecena (10.72%), Dodecanal (4.1%), dodecan-1-ol (3.13%) Bhuiyan et al (2009) [12]: 2-decenoic acid (30.8%), E-11-tetradecenoic acid (13.4%), capric acid (12.7%), undecyl alcohol (6.4%) and tridecanoic acid (5.5%), undecanoic acid (2.1%), 2-dodecanal (1.3%), 2-undecenal (3.9%), cyclododecane (2.5%), decamethylene glycol (1.2%), decanal (1.4%) and dodecanoic acid (2.6%). Kumar et al. (2022) [13]: Decanal, (E)-2-decenal, (E)-2-decenol, 1-decanol, dodecanal, (E)-2-dodecenal, and (E)-2-tetradecenal, with relative abundances varying by cultivars. |
Dill (Anethum graveolens) [14]
Dill seed
Primary aroma compounds: Carvone
Other significant aroma compounds:
Coumarins, flavonoids, phenolic acids, and steroids
Dill herb
Primary aroma compounds: α-phellandrene, limonene, dill ether, myristicin.
Primary aroma compounds: Carvone
Other significant aroma compounds:
Coumarins, flavonoids, phenolic acids, and steroids
Dill herb
Primary aroma compounds: α-phellandrene, limonene, dill ether, myristicin.
Hops [15] [16] [17]
Primary aroma compounds
Terpenes of:
•ẞ-pinene: Spicy, piney [17]
•Myrcene: Green, herbaceous, piney, hoppy, resinous [16]
•Linalool: Floral, rose-like, orange [16]
•Caryophyllene: Woody, spice, herbal [16]
•Farnesene: Floral [17]
•Humulene: Woody, spicy, piney, hoppy [16]
•Geraniol: Fruity, floral, waxy [16]
In an upcoming series on brewing and distilling grain, we will have an issue dedicated to hops.
Terpenes of:
•ẞ-pinene: Spicy, piney [17]
•Myrcene: Green, herbaceous, piney, hoppy, resinous [16]
•Linalool: Floral, rose-like, orange [16]
•Caryophyllene: Woody, spice, herbal [16]
•Farnesene: Floral [17]
•Humulene: Woody, spicy, piney, hoppy [16]
•Geraniol: Fruity, floral, waxy [16]
In an upcoming series on brewing and distilling grain, we will have an issue dedicated to hops.
Lemongrass (Cymbopogon spp)
|
Common cultivars
The lemongrass (Cymbopogon) genus includes ~180 species including: •West Indian lemongrass Cymbopogon citratus •East-Indian lemongrass Cymbopogon flexuosus •Citronella grass Cymbopogon nardus •Java citronella Cymbopogon winterianus •Palmarosa: Cymbopogon martinii •Barbed wire grass Cymbopogon refractus Primary Aroma compounds ( 60–80% of Lemongrass Essential Oilo) •Citral: This mixture of the stereoisomers geranial and neral can be used as a quality marker for lemongrass essential oil. •Neral, isoneral, geranial, isogeranial, geraniol, geranyl acetate, citronellal, citronellol, germacrene-D, and elemol. •Other significant aroma compounds •Camphene, pinene, limonene, linalool, citronellyl acetate, elemene, and caryophyllene oxide. |
Aroma Compounds in Specific Species [19]
Cymbopogon citratus Saleem et al (2003) [20]: Citral a (40.8%), Citral b (32%), Nerol (4.18%), α-Pinene (0.07%), β-Pinene (0.04%), Myrecene (0.72%), α-Terpinol (0.45%), Terpinolene (1.23%), Geranyl acetate (0.83%), Citronellol (2.10%), and Geraniol (3.04%). Chanthai et al (2012) [21]: Geranial/trans-citral (49.4%), Neral/cis-citral (27.48%), Myrcene (9.73%), geraniol (3.23%), α-thujene (.9%), cis-β-ocimene (1.22%), trans-β-ocimene (1.03%), citronellal (2.72), limonene oxide (1.50%), citronellol (0.59%), α-copaene (.38%), and humulene (1.81%). Cymbopogon flexuosus (East-Indian lemongrass) [19]: Rich in geranial (42%) and neral (33%) which are together called citral 42+33=73%) with relatively trace amounts of geraniol and geranyl acetate. Cymbopogon martinii (Palmarosa) Dominated by geraniol (71%) and geranyl acetate (5%) rather than aldehydes like neral and geranial [19]. Cymbopogon winterianus (Java citronella): Citronellal (39%) represents the principal constituent followed by 21% of alcohols (geraniol and citronellol) and 11% of acetates (geranyl acetate and citronellyl acetate) [19]. Cymbopogon nardus (Citronella grass) Contains α-pinene (1.14%), citronellal (.63%), limonene oxide (.47%), citronellol (4.33%), geraniol (55.57%), neral/cis-citral (8.34%), geranial/trans-citral (55.57%), eugenol (2.51%), humulene (1.63%), α-copaene (6.61%), β-caryophyllene (2.32%), germacrene D (.2%), α-cubebene (1.23%), γ-cadinene and δ-cadinene (4.26%), and δ-cadinol (.58%) [21]. |
Mint [22]
Common cultivars
Main and commercially important mint species [23]
•Peppermint (Mentha × piperita L.). Black Mitcham is the primary cultivar. Mentha × piperita L. a hybrid of Mentha aquatica and Mentha spicata
•Arvensis/Corn Mint (Mentha arvensis): The source of commercial natural menthol
•Spearmint (Mentha spicata L.) primary cultivars
•Native spearmint a hybrid of Mentha longifolia and Mentha suaveolens
•Scotch spearmint is a hybrid of Mentha arvensis and Mentha spicata.
Other Common Species
•Water mint (Mentha aquatic)
•Apple mint (Mentha suaveolens)
•Chocolate mint (Mentha x piperita ‘Chocolate’)
•Pineapple mint (Mentha suaveolens ‘Variegata’)
•Horsemint (Mentha longifolia)
•Eau de cologne mint/Orange Mint (Mentha x piperita f. citrate)
•Pennyroyal mint (Mentha pulegium),
General mint aroma compounds
Fresh Leaves [25]:
Peppermint: (–)–menthol, menthone, menthyl acetate, and (+)–menthofuran.
Dried leaves [22]:
Eucalyptol, (±)camphorquinone, and menthol.
Essential oil [26]:
Menthol, Menthone, Menthyl acetate, Limonene, Carvone.
Main and commercially important mint species [23]
•Peppermint (Mentha × piperita L.). Black Mitcham is the primary cultivar. Mentha × piperita L. a hybrid of Mentha aquatica and Mentha spicata
•Arvensis/Corn Mint (Mentha arvensis): The source of commercial natural menthol
•Spearmint (Mentha spicata L.) primary cultivars
•Native spearmint a hybrid of Mentha longifolia and Mentha suaveolens
•Scotch spearmint is a hybrid of Mentha arvensis and Mentha spicata.
Other Common Species
•Water mint (Mentha aquatic)
•Apple mint (Mentha suaveolens)
•Chocolate mint (Mentha x piperita ‘Chocolate’)
•Pineapple mint (Mentha suaveolens ‘Variegata’)
•Horsemint (Mentha longifolia)
•Eau de cologne mint/Orange Mint (Mentha x piperita f. citrate)
•Pennyroyal mint (Mentha pulegium),
General mint aroma compounds
Fresh Leaves [25]:
Peppermint: (–)–menthol, menthone, menthyl acetate, and (+)–menthofuran.
Dried leaves [22]:
Eucalyptol, (±)camphorquinone, and menthol.
Essential oil [26]:
Menthol, Menthone, Menthyl acetate, Limonene, Carvone.
Peppermint (Mentha piperita)
|
Content plant volatiles in dried leaves: 98.27%
Primary constituents in dried leaves: Park et al. (2016) [22]: Eucalyptol (62.16%) is dominant and 4-terpineryl acetate (6.17%), caryophyllene (5.50%), menthol (4.30%) are accumulated slightly in peppermint. Sun et al. (2014) [27]: Menthol (30.69%), menthone (14.51%), and menthy acetate (12.86%). Buleandra et al (2016) [26] Dried leaves: Menthone (25.4%), eucalyptol (17.7%), and menthol (12.1%) Essential oil: Menthol (46.8%) and menthone (25.6%) Peters et al (2021) [25]: •Black Mitcham oils were characterized by the highest contents of menthone, neomenthol, 1,8–cineole, (+)‑menthofuran, menthalactone compared to the other three analyzed mint species. •The main aroma compounds in fresh Black Mitcham are menthol, menthone , (+)‑menthofuran, neomenthol, and 1,8–cineole. Schmidt et al (2009) [28]: Main constituents: menthol (40.7%) and menthone (23.4%). Other significant components: (+/-)-menthyl acetate, 1,8-cineole, limonene, β-pinene, and β-caryophyllene. Spearmint (Mentha spicata) Content of plant volatiles in dried leaves: 97.42% Primary constituents in dried leaves: Park et al. (2016) [22]: Eucalyptol (46.28%), 1,3,5-tri-Methyl-6-methylene-cyclohexene (19.25%), and caryophyllene (5.47%). Soković et al. (2009) [29]: Carvone (49.5%) and menthone (21.9%) as the main volatiles. Buleandra et al (2016) [26] •Dried leaves: Limonene (37.0%), carvone (13.0%), β-pinene (10.4%), and α-pinene (9.8%). •Dried leaf essential oil: Carvone (51.7%), dihydrocarveol (11.5%), and cis-dihydrocarvone (9.1%). Peters et al (2021) [25]: •Scotch and Native spearmint main aroma compounds: (R,S)‑carvone, limonene, 1,8–cineole, and myrcene. •Native spearmint oils showed the highest concentration of carbonyl compounds like nonanal, isovalerate, (E,Z)‑2,6‑nonadienal, a‑ionone, and alcohols like pinocarveol, dihydrocarveol, octanol, pentanol. •Scotch spearmint showed high amounts o (R,S)‑carvone, 2-phenylethanol, p-cresol, terpinolene, and limonene. Kelley et al (2017) [30]: •(R)-(−)-carvone was the most potent odorant in all three spearmint oils. Additional predominant odorants in all spearmint oils included eugenol, ethyl (S)-(+)-2-methylbutanoate, (E)-β-damascenone, and (3E,5Z)-1,3,5-undecatriene. •Among the compounds quantitated, those with the highest OAVs were (R)-(−)-carvone, 1,8-cineole, (E,Z)-2,6-nonadienal, (E)-β-damascenone, and (3E,5Z)-1,3,5-undecatriene. |
Other Species
Arvensis/Corn Mint (Mentha arvensis): •Peters et al (2021)[25]: Quantitative dominance of menthone and menthol. Compounds that were also present in high concentration: 1,8–cineole, neomenthol, α–humulene, α–pinene. It also contained the highest amounts of Linalool, geraniol, α–terpineol, pinocarveol, and neomenthol relative to the other species studied by Peters et al (2021). Water mint (Mentha aquatic) •Content of plant volatiles in dried leaves: 94.95% •Primary constituents in dried leaves Park et al. (2016) [22]: (−)-calamenene (12.17%), eucalyptol (11.39%), citronella (11.04%), and α-gurjunnene (10.88%). Apple mint (Mentha suaveolens) •Content of plant volatiles in dried leaves: 98.54% •Primary constituents in dried leaves Park et al. (2016) [22]: (±)camphorquinone (51.61%), eucalyptol (19.49%), and γ-terpinene (5.25%) Chocolate mint (Mentha x piperita ‘Chocolate’) •Content of plant volatiles in dried leaves: 99.70%; Primary constituents in dried leaves: •Park et al. (2016) [22]:Menthol (36.31%), eucalyptol (22.70%), and D-limonene (7.91%). Park et al. (2016) notes that chocolate mint contained the highest concentration of menthol relative to any mint tested in their study. Pineapple mint (Mentha suaveolens ‘Variegata’) •Content of plant volatiles in dried leaves: 97.02% •Primary constituents in dried leaves: •Park et al. (2016) [22]: Eucalyptol (37.36%), γ-terpinene (16.94%), 4-terpineryl acetate (10.53%). Horsemint (Mentha longifolia) •Content of plant volatiles in dried leaves: 99.70% •Primary constituents in dried leaves: •Park et al. (2016) [22]: p-menthan-3-one (31.24%), eucalyptol (9.35%), 1,3-diethylbenzene (6.02%), and thymol (5.41%). •Koliopoulos et al. (2010) [31]: •M. longifolia from central Greece: Piperitone oxide (33.4%), 1,8-cineole 24.5%, and trans-piperitone epoxide (17.4%) from central Greece. •M. longifolia from East-Southern Greece: Carvone (54.7%), limonene (20.0%), β-pinene, and piperitone (5.0%, respectively). Eau de cologne mint/Orange Mint (Mentha x piperita f. citrate) •Content of plant volatiles in dried leaves: 97.84% yielded the highest essential oil content •Primary constituents in dried leaves: Park et al. (2016) [22]: Eucalyptol (18.36%), caryophyllene (9.81%), p-methan-3-one (8.13%), 1,3-cyclohexandiene (6.40%), 1-methyl-4-(methylidene)cyclohexane (6.31%), and 2,4,6-oxtatriene (6.28%). Pennyroyal mint (Mentha pulegium) •Content of plant volatiles in dried leaves: 99.81%. •Primary constituents in dried leaves: Park et al. (2016) [2]: p-methan-3-one (71.87%) and menthol (11.29%). |
For more insight
Park, Y. J., Baskar, T. B., Yeo, S. K., Arasu, M. V., Al-Dhabi, N. A., Lim, S. S., & Park, S. U. (2016). Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus, 5, 1-10. doi.org/10.1186/s40064-016-3283-1
Buleandra, M., Oprea, E., Popa, D. E., David, I. G., Moldovan, Z., Mihai, I., & Badea, I. A. (2016). Comparative chemical analysis of Mentha piperita and M. spicata and a fast assessment of commercial peppermint teas. Natural product communications, 11(4), www.journals.sagepub.com/doi/pdf/10.1177/1934578X1601100433
Park, Y. J., Baskar, T. B., Yeo, S. K., Arasu, M. V., Al-Dhabi, N. A., Lim, S. S., & Park, S. U. (2016). Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus, 5, 1-10. doi.org/10.1186/s40064-016-3283-1
Buleandra, M., Oprea, E., Popa, D. E., David, I. G., Moldovan, Z., Mihai, I., & Badea, I. A. (2016). Comparative chemical analysis of Mentha piperita and M. spicata and a fast assessment of commercial peppermint teas. Natural product communications, 11(4), www.journals.sagepub.com/doi/pdf/10.1177/1934578X1601100433
Oregano
|
Common cultivars [32]
Oregano is a generic name for a group of plants consisting of six botanical families, at least 61 species, and 17 genera. Common cultivars include: •Common Oregano: Origanum vulgare •Greek Oregano: Origanum vulgare subsp. hirtum (Origanum hirtum) •Italian Oregano: Origanum x majoricum •Sweet Marjoram: Origanum majorana •Cretan dittany/Hop marjoram: Origanum dictamnus •Mexican Oregano: Lippia graveolens •Turkish oregano: Origanum onites •Spanish oregano: Coridohymus capitatu General Aroma Compounds [32] Principal terpenes Carvacrol, thymol, γ-terpinene, and p-cymenel Other significant terpenes Terpinen-4-ol, linalool, β-myrcene, trans-sabinene hydrate, and β-caryophyllene. Common Oregano (Origanum vulgare) Principal Aroma Compounds Thymol, carvacrol [33] Other signficant aroma compounds Lombrea et al (2020) [34]: p-cymene, γ-terpinene, and linalool. Teixeira et al 2013 [35] •Oxygenated monoterpenes: β-fenchyl alcohol (12.8%), and δ-terpineol (7.5%) were the most abundant. •Monoterpene hydrocarbons (26.4%): γ-terpinene (11.6%) and α-terpinene (3.7%) were the most abundant. •1-methyl-3-(1-methylethyl)-benzene (6.8%) was also substantial. Primary chemotypes as noted by Lukas et al (2015) and Lombrea et al (2020) vary by cultivar and growing location/climate factors, and are correlated with the amount of essential oil produced as [36]: |
High essential oil (2%+) Origanum vulgare subspecies like hirtum, glandulosum, gracile)
Cymyl-compounds: Often the chemotype of cultivars that are rich in essential oils usually accumulate large amounts of phenolic monoterpenes deriving from the ‘cymyl’-pathway (mainly carvacrol and/or thymol and their biosynthetic precursors γ-terpinene and p-cymene. Essential oil-poor plants The monoterpene fraction is usually poor in ‘cymyl’-compounds and relatively higher in one or both of the following, with the highest amount being the chemotype: •Sabinyl-compounds: Bicyclic ‘sabinyl’-compounds of mainly sabinene and cis-/trans-sabinene hydrate and their acetates. •Linalool/linalyl acetate: Acyclic compounds mainly of linalool but also linalyl acetate, β-ocimene, or myrcene. •These are also accompanied by bornane type compounds (e.g. camphor, borneol, bornyl acetate) and is often accompanied by high amounts of sesquiterpenes (such as e.g. β-caryophyllene, germacrene D, bicyclogermacrene, α- and γ-muurolene, β-caryophyllene oxide). Influence of environmental factors: The essential oil composition varies according to environmental factors, geographical area, harvesting time, and stage of plant maturity, with the chemotype composition being in direct correlation with the climatic factors. Lombrea et al 2020 noted plants from the Mediterranean climate present an active cymyl- and/or linalool pathway, while plants growing in areas characterized by Continental climate are poorer in monoterpenes and display a more active sabinyl-pathway for example: •Plants from Italy: Thymol, carvacrol, linalyl acetate, and γ-terpinene •Plants from Portugal: Carvacrol, thymol, γ-terpinene, and β-fenchyl alcohol. •Plants from Montenegro had germacrene D, β-caryophyllene, linalyl acetate, and α-terpineol as major constituents. Mexican Oregano (Lippia graveolens) [37 [38] Primary Aroma compounds Carvacrol and thymol Other significant compounds β-caryophyllene, p-cymene, α-humelene, 1,8-Cineole, γ-Terpinene |
Parsley
Flat Leaf/Italian Petroselinum crispum var neapolitanum
Primary Aroma Compounds
Petropoulos et al (2010) [39]:
The characteristic aroma of fresh parsley is due to β-phellandrene,1,3,8-p-menthatriene, p-α-dimethylstyrene and terpinolene, apiole, myristicin, and myrcene.
Farouk et al. (2017) [40]
•Egyptian grown parsley (essential oil from fresh leaf): Myristicin (26.41%), β-phellanderene (11.61%), α-phellandrene (10.54%), 1,3,8-p-menthatriene (9.41%), p-cymene (8.63%), myrcene (6.12%), α-pinene(1.79%), and p-cymene (1.09%).
•Egyptian parsley (dried): 1,3,8-p-Menthtriene (39.76%), myrcene (20.07%), and α-phellandrene (19.11%) were the predominant volatile compounds of
•Madinah parsley (essential oil from fresh leaf): Myristicine (20.7%), β-phellandrene (17.45%), myrcene (11.42%), p-cymene (8.95%), and 1,3,8-p-menthtriene (6.64%). It was noted that some compounds could be identified only in the Madinah parsley essential oil, such as cis-pinocarveol (2.8%), p-methyl guaiacol (2.14%), 2-bornanol, 2-methyl (4.09%), and linalool (0.76%).
•Madinah parsley (dried): β-phellandrene (31.83%), 1,3,8-p-menthatriene (29.1%), and myrcene (26.42%).
Masanetz and Grosch (1998) [41]:
The most potent odorants of parsley which create its characteristic impact flavor were reported as p-1,3,8-menthatriene, myrcene, 2-sec-butyl-3-methoxypyrazine, myristicin, linalool, 6-decenal, and 3-hexenal.
Other Significant Compounds:
Azis et al (2013) [42]: Apiole and Limonene
Primary Aroma Compounds
Petropoulos et al (2010) [39]:
The characteristic aroma of fresh parsley is due to β-phellandrene,1,3,8-p-menthatriene, p-α-dimethylstyrene and terpinolene, apiole, myristicin, and myrcene.
Farouk et al. (2017) [40]
•Egyptian grown parsley (essential oil from fresh leaf): Myristicin (26.41%), β-phellanderene (11.61%), α-phellandrene (10.54%), 1,3,8-p-menthatriene (9.41%), p-cymene (8.63%), myrcene (6.12%), α-pinene(1.79%), and p-cymene (1.09%).
•Egyptian parsley (dried): 1,3,8-p-Menthtriene (39.76%), myrcene (20.07%), and α-phellandrene (19.11%) were the predominant volatile compounds of
•Madinah parsley (essential oil from fresh leaf): Myristicine (20.7%), β-phellandrene (17.45%), myrcene (11.42%), p-cymene (8.95%), and 1,3,8-p-menthtriene (6.64%). It was noted that some compounds could be identified only in the Madinah parsley essential oil, such as cis-pinocarveol (2.8%), p-methyl guaiacol (2.14%), 2-bornanol, 2-methyl (4.09%), and linalool (0.76%).
•Madinah parsley (dried): β-phellandrene (31.83%), 1,3,8-p-menthatriene (29.1%), and myrcene (26.42%).
Masanetz and Grosch (1998) [41]:
The most potent odorants of parsley which create its characteristic impact flavor were reported as p-1,3,8-menthatriene, myrcene, 2-sec-butyl-3-methoxypyrazine, myristicin, linalool, 6-decenal, and 3-hexenal.
Other Significant Compounds:
Azis et al (2013) [42]: Apiole and Limonene
French/Curley Parsley Petroselinum crispum var. crispum
Curly Leaf Parsley Aroma Compounds
Aziz et al (2013) [43]: Myristcin (25.70 to 46.41%), followed by β-phellandrene (8.77 to 16.42%), β-myrcene (6.72 to 11.44%), p-men-tha-1,3,8-triene (0.89 to 12.83%), p-cymene (5.14 to 8.16%) and alpha-terpinolene (3.32 to 7.91%). Farouk et al (2017) notes that the absence of 1,3,8-p-menthatriene is likely due to differences between winter and summer parsley [42].
Curly Leaf Parsley Aroma Compounds
Aziz et al (2013) [43]: Myristcin (25.70 to 46.41%), followed by β-phellandrene (8.77 to 16.42%), β-myrcene (6.72 to 11.44%), p-men-tha-1,3,8-triene (0.89 to 12.83%), p-cymene (5.14 to 8.16%) and alpha-terpinolene (3.32 to 7.91%). Farouk et al (2017) notes that the absence of 1,3,8-p-menthatriene is likely due to differences between winter and summer parsley [42].
Rosemary (Rosmarinus officinalis L., Lamicaeae)
Rosemary (Rosmarinus officinalis L., Lamicaeae)
Primary Aroma Compounds [44]
Rosemary essential oil: α-pinene, eucalyptol (1,8-cineole), and camphor.
Other significant aroma compounds
Primary Aroma Compounds [44]
Rosemary essential oil: α-pinene, eucalyptol (1,8-cineole), and camphor.
Other significant aroma compounds
- Pellegrini et al (2018) [44]: Verbenone, borneol, camphor, and bornyl acetate.
- Tschiggerl and Bucar (2010) [45]:
- Rosemary hydrodistillation/SPE: 1,8-cineole (42.4%/44.7%), cam- phor (31.4%/31.8%), α-terpineol (8.6%/8.1%) and borneol (8.3%/7.8%).
- Essential oil of the leaves: 1,8-cin- eole (41.6%), camphor (17.0%), α-pinene (9.9%), α-terpineol (4.9%), and borneol (4.8%).
- Micić et al. (2021) [46]:
- Camphene, β-pinene, limonene, p-cymene, borneol, bornyl acetate, and trans-β-caryophyllene.
- Serbian-grown rosemary and not Russian-grown rosemary were found to contain α-terpineol and terpinen-4-ol, γ-terpinene, α- and β-phellandrenes, and terpinolene.
- Russian-grown rosemary and not Serbian-grown rosemary were found to contain: m-cymene, α-pinane oxide, pinocarvone, and carvone.
- Karkaya (2014) [47]:
- α-terpineol (35– 41 mg/mL), borneol (35–40 mg/mL), β-pinene (~33 mg/mL), and camphene (~25 mg/mL).
Sage/ Common Sage
(Salvia officinalis)
Primary Aroma Compounds
Camphor. The essential oil of sage contains about 20% camphor Hamidpour et al. (2014) [48.
Secondary Aroma Compounds
Camphor. The essential oil of sage contains about 20% camphor Hamidpour et al. (2014) [48.
Secondary Aroma Compounds
- Hamidpour et al. (2014): 1,8-cineole, borneol, bornyl acetate, camphene,α -thujone and β-thujone, linalool, α- and β-caryophyllene, α-humulene, α- and β-pinene, viridiflorol, pimaradiene, salvianolic acid, rosmarinic acid, carno- solic acid, ursolic acid, etc.
- Jonas et al (2021) [49]
- Fresh Sage: (Z)-3-hexenal, 1,8-cin- eol, borneol, and eugenol, where the highest OAVs were myrcene, (Z)-3-hexenal, (1S,2R,4S)-borneol and 1,8-cineol.
- Influence of drying
- Monoterpenes remained nearly unchanged during drying, whereas highly volatile compounds like dimethyl sulfide or 2- and 3-methylbutanal decreased.
- Almost a total loss occurred for 3-(methylthio)propanal, phenyl- acetaldehyde, and (Z)-3-hexenal.
- Monoterpenes remained nearly unchanged during drying, whereas highly volatile compounds like dimethyl sulfide or 2- and 3-methylbutanal decreased.
- Fresh Sage: (Z)-3-hexenal, 1,8-cin- eol, borneol, and eugenol, where the highest OAVs were myrcene, (Z)-3-hexenal, (1S,2R,4S)-borneol and 1,8-cineol.
Shiso (Perilla frutescens var. crispa) and Perilla frutescens (L.) Britt.
Common Cultivars [50]
Perilla frutescens var. crispa
Kketnip/ Korean perilla: Perilla frutescens (L.) Britt.
Primary Aroma Compounds [51]
There are seven chemotypes (plant subspecies with the same morphological/structural characteristics but produce different quantities of chemical components in their essential oils). The primary edible cultivars and their relevant chemotypes are:
Perilla frutescens var. crispa PA-type primarily contains: 1,8-p-menthadiene-7-al (50%), Peril alcohol (1%), Limonene (15-20%).
Kketnip/ Korean perilla: Perilla frutescens (L.) Britt
PK-type primarily contains: Perillaketone (3-(4-methyl-1-oxopentyl)
furan) and/or isoegomaketone (3-4-methyl-1-oxo-2-penten).
Secondary Aroma Compounds [52]
Geraniol, Citral, Caryophyllene and Farnesene.
Perilla frutescens var. crispa
- P. frutescens var. crispa is smaller in plant height and seed size (below 2 mm) and has hard seeds, red or green coloration in the leaves and stem, wrinkly or non-wrinkly leaves, and a distinctive fragrance (Lee et al., 2002).
- Japanese cultivars (‘Ao Shiso’ and ‘Aka Shiso’, Tokita, ‘Qing Su’ and ‘Purple Zi Su’, Agrohaitai) are of the PA-chemotype as they produce almost exclusively perillaldehyde. The green cultivars presented a significantly higher content of perillaldehyde than the red cultivars.
Kketnip/ Korean perilla: Perilla frutescens (L.) Britt.
- In Korea, only var. frutescens of the PK-chemotype (which con- tains perillaketone instead of perillaldehyde) is used both as an oil and vegetable crop.
- P. frutescens var. frutescens generally is taller, and larger in seed size (above 2 mm) and has soft seeds, green leaves and stems, and non-wrinkly leaves with a specific fragrance.
- Perilla cultivars are usually distinguished on the basis of the green or red colour of the leaves, irrespective of the sub-species; red leaves contain a large number of anthocyanins and are com- monly used as food colorants.
Primary Aroma Compounds [51]
There are seven chemotypes (plant subspecies with the same morphological/structural characteristics but produce different quantities of chemical components in their essential oils). The primary edible cultivars and their relevant chemotypes are:
Perilla frutescens var. crispa PA-type primarily contains: 1,8-p-menthadiene-7-al (50%), Peril alcohol (1%), Limonene (15-20%).
Kketnip/ Korean perilla: Perilla frutescens (L.) Britt
PK-type primarily contains: Perillaketone (3-(4-methyl-1-oxopentyl)
furan) and/or isoegomaketone (3-4-methyl-1-oxo-2-penten).
Secondary Aroma Compounds [52]
Geraniol, Citral, Caryophyllene and Farnesene.
Tarragon (Artemisia dracunculus)
Primary Aroma Compounds [53]
Estragole/methyl chavicol/p-allylanisole, Sabine, Methyl eugenol, Elemicin
Secondary Aroma Compounds
Terpinen-4-ol, β-ocimene, cis-ocimene, α-trans-ocimene, limonene and trans-anethole, α-phellandrene, β-phellandrene, (Z)-artemidin, capillene.
Estragole/methyl chavicol/p-allylanisole, Sabine, Methyl eugenol, Elemicin
Secondary Aroma Compounds
Terpinen-4-ol, β-ocimene, cis-ocimene, α-trans-ocimene, limonene and trans-anethole, α-phellandrene, β-phellandrene, (Z)-artemidin, capillene.
Thyme
Common Cultivars
Primary Aroma Compounds
Thymol, carvacrol, γ-terpinene, and p-cymene
Secondary Aroma Compounds
Thyme (Thymus vulgaris L)
Thyme - Lemon (Thymus × citriodorus) [58]
- Thyme (Thymus vulgaris L)
- Thyme - Lemon (Thymus × citriodorus)
Primary Aroma Compounds
Thymol, carvacrol, γ-terpinene, and p-cymene
Secondary Aroma Compounds
Thyme (Thymus vulgaris L)
- Sonmezdag et al (2016) [54]: β-pinene, β-caryophyllene, 1-borneol, 1,8-cineole.
- Hudaib and Aburjai (2007) [55]: p-cymene (3.4–8.2%), γ-terpinene (1.6–7.7%), 1,8-cineole (up to 2.1%), α-thujone (up to 1.2%), camphor (up to 1.1%) and β-caryophyllene (0.2–2.8%).
- Calín-Sánchez et al. (2013) [56]: γ-terpinene, p-cymene, c-terpinene, caryophyllene, and α-terpinene.
- Lee et al. (2005) [57]: Linalool (4.0%), α-terpineol (2.4%), 1,8-cineole (2.1%), Borneol (2.0%).
Thyme - Lemon (Thymus × citriodorus) [58]
- Primary Aroma Compounds (Essential oil): Geraniol, nerol, geranial, and neral
- Secondary Aroma Compounds : None mentioned
Sources and Suggested Reading
1. Jonas, M., & Schieberle, P. (2021). Characterization of the key aroma compounds in fresh leaves of garden sage (Salvia officinalis L.) by means of the sensomics approach: influence of drying and storage and comparison with commercial dried sage. Journal of Agricultural and Food Chemistry, 69(17), 5113-5124. https://pubs.acs.org/doi/10.1021/acs.jafc.1c01275
2. De Martino L, Caputo L, Amato G, Iannone M, Barba AA, De Feo V. Postharvest Microwave Drying of Basil (Ocimum basilicum L.): The Influence of Treatments on the Quality of Dried Products. Foods. 2022; 11(7):1029. https://doi.org/10.3390/foods11071029
3. Lee, S. J., Umano, K., Shibamoto, T., & Lee, K. G. (2005). Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food chemistry, 91(1), 131-137. https://www.researchgate.net/publication/238378687_Identification_of_volatile_components_in_basil_L_ and_thyme_leaves_L_and_their_antioxidant_properties
4. Tangpao T, Chung H-H, Sommano SR. Aromatic Profiles of Essential Oils from Five Commonly Used Thai Basils. Foods. 2018; 7(11):175. https://doi.org/10.3390/foods7110175 5. Sellami, I. H., Wannes, W. A., Bettaieb, I., Berrima, S., Chahed, T., Marzouk, B., & Limam, F. (2011). Qualitative and quantitative changes in the essential oil of Laurus nobilis L. leaves as affected by different drying methods. Food chemistry, 126(2), 691-69. https://www.sciencedirect. com/science/article/abs/pii/S0308814610014299
6. Kilic, A., Hafizoglu, H., Kollmannsberger, H., & Nitz, S. (2004). Volatile constituents and key odorants in leaves, buds, flowers, and fruits of Laurus nobilis L. Journal of agricultural and food chemistry, 52(6), 1601-1606. Retrieved from www.academia.edu/741769/Volatile_constituents_and_key_odorants_in_ leaves_buds_flowers_and_fruits_of_Laurus_nobilis_L
7. Wikipedia contributors. (2023, May 23). Bay leaf. In Wikipedia, The Free Encyclopedia. Retrieved 10:39, June 17, 2023, from https://en.wikipedia. org/w/index.php?title=Bay_leaf&oldid=1156462697
8. Mandal, S., & Mandal, M. (2015). Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine, 5(6), 421-428. https://www. sciencedirect.com/science/article/pii/S2221169115000647
9. Łyczko, J.; Masztalerz, K.; Lipan, L.; Iwiński, H.; Lech, K.; Carbonell- Barrachina, Á.A.; Szumny, A. Coriandrum sativum L.—Effect of Multiple Drying Techniques on Volatile and Sensory Profile. Foods 2021, 10, 403. https://doi.org/10.3390/foods10020403
10. Shahwar, M. K., El-Ghorab, A. H., Anjum, F. M., Butt, M. S., Hussain, S., & Nadeem, M. (2012). Characterization of coriander (Coriandrum sativum L.) seeds and leaves: volatile and non volatile extracts. International journal of food properties, 15(4), 736-747. https://doi.org/10.1080/10942912.2010.500068
11. Mandal, S., & Mandal, M. (2015). Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine, 5(6), 421-428. https://www.sciencedirect.com/science/article/pii/S2221169115000647
12. Bhuiyan, M. N. I., Begum, J., & Sultana, M. (2009). Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. ||| Bangladesh Journal of Pharmacology|||, 4(2), 150-153. http://bdpsjournal.org/index.php/bjp/article/view/395
13. Kumar S, Ahmad R, Saeed S, Azeem M, Mozūraitis R, Borg-Karlson A-K and Zhu G (2022) Chemical Composition of Fresh Leaves Headspace Aroma and Essential Oils of Four Coriander Cultivars. Front. Plant Sci. 13:820644. https://doi.org/10.3389/fpls.2022.820644
14. Jana, S., & Shekhawat, G. S. (2010). Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacognosy reviews, 4(8), 179–184. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3249919/
15. Jensen, P., Tielkemeier, S., & Zeigler, N. (2020, May). Yakima Chief Hops: Getting the Most from your Hops. Brewers Association. https://www.brewersassociation. org/wp-content/uploads/2020/05/CBC-Online- Seminar-Presentation-Getting-the-Most-from- Your-Hops-Presented-by-Yakima-Chief-Hops.pdf
16. Kemp, O., Hofmann, S., Braumann, I., Jensen, S., Fenton, A., & Oladokun, O. (2021). Changes in key hop-derived compounds and their impact on perceived dry-hop flavour in beers after storage at cold and ambient temperature. Journal of the Institute of Brewing, 127(4), 367-384. https://onlinelibrary.wiley.com/doi/full/10.1002/jib.667
17. Hieronymus, S. (2016, March 16). Hops Oils & Aroma: Uncharted Waters. Craft Beer & Brewing. Retrieved June 28, 2023, from https:// beerandbrewing.com/hops-oils--aroma-uncharted-waters/
18. Mukarram M, Choudhary S, Khan MA, Poltronieri P, Khan MMA, Ali J, Kurjak D, Shahid M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants. 2022; 11(1):20. https://doi.org/10.3390/antiox11010020
19. Meena, S.; Kumar, S.R.; Rao, D.K.V.; Dwivedi, V.; Shilpashree, H.B.; Rastogi, S.; Shasany, A.K.; Nagegowda, D.A. De Novo sequencing and analysis of lemongrass transcriptome provide first insights into the essential oil biosynthesis of aromatic grasses. Front. Plant Sci. 2016, 7, 1129. https://doi.org/10.3389/fpls.2016.01129
20. Saleem M, Afza N, Anwar MA, Hai SM, Ali MS. Comparative study of essential oils of Cymbopogon citratus and some members of the Genus Citrus. Nat Prod Res. 2003;17:369–73. Retrieved from: https://www.researchgate.net/publication/9065118_A_Comparative_Study_of_Essential_Oils_of_Cymbopogon_ Citratus_and_Some_Members_of_the_Genus_Citrus
21. Chanthai, S., Prachakoll, S., Ruangviriyachai, C., & Luthria, D. L. (2012). Influence of extraction methodologies on the analysis of five major volatile aromatic compounds of citronella grass (Cymbopogon nardus) and lemongrass (Cymbopogon citratus) grown in Thailand. Journal of AOAC International, 95(3), 763-772. https://doi.org/10.5740/jaoacint.11-335
22. Park, Y. J., Baskar, T. B., Yeo, S. K., Arasu, M. V., Al-Dhabi, N. A., Lim, S. S., & Park, S. U. (2016). Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus, 5, 1-10. https://doi.org/10.1186/s40064-016-3283-1
23. Vining, K. J., Hummer, K. E., Bassil, N. V., Lange, B. M., Khoury, C. K., & Carver, D. (2020). Crop wild relatives as germplasm resource for cultivar improvement in mint (Mentha L.). Frontiers in Plant Science, 11, 1217. https:// www.frontiersin.org/articles/10.3389/fpls.2020.01217/full
24. Srivastava, R. K., Singh, A. K., Kalra, A., Tomar, V. K. S., Bansal, R. P., Patra, D. D., ... & Kumar, S. (2002). Characteristics of menthol mint Mentha arvensis cultivated on industrial scale in the Indo- Gangetic plains. Industrial crops and products, 15(3), 189-198.
25. Peters, V. C. T., Dunkel, A., Frank, O., McCormack, B., Dowd, E., Didzbalis, J., ... & Hofmann, T. (2021). A high throughput toolbox for comprehensive flavor compound mapping in mint. Food Chemistry, 365, 130522. https://doi.org/10.1016/j.foodchem.2021.130522.
26. Buleandra, M., Oprea, E., Popa, D. E., David, I. G., Moldovan, Z., Mihai, I., & Badea, I. A. (2016). Comparative chemical analysis of Mentha piperita and M. spicata and a fast assessment of commercial peppermint teas. Natural product communications, 11(4), 1934578X1601100433. https:// journals.sagepub.com/doi/pdf/10.1177/1934578X1601100433
27. Sun, Z., Wang, H., Wang, J., Zhou, L., & Yang, P. (2014). Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PloS one, 9(12), e114767. https://journals. plos.org/plosone/article?id=10.1371/journal.pone.0114767
28. Schmidt, E., Bail, S., Buchbauer, G., Stoilova, I., Atanasova, T., Stoyanova, A., ... & Jirovetz, L. (2009). Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha x piperita. Natural product communications, 4(8). https:// journals.sagepub.com/doi/pdf/10.1177/1934578X0900400819
29. Soković MD, Vukojević J, Marin PD, Brkić DD, Vajs V, Van Griensven LJ (2009) Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 14:238–249. https://doi.org/10.3390/molecules14010238
30. Kelley, L. E., & Cadwallader, K. R. (2017). Identification and quantitation of potent odorants in spearmint oils. Journal of agricultural and food chemistry, 66(10), 2414- 2421. https://pubs.acs.org/doi/abs/10.1021/acs.jafc.6b04852
31. Koliopoulos G, Pitarokili D, Kioulos E, Michaelakis A, Tzakou O (2010) Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the west nile virus mosquito Culex pipiens. Parasitol Res 107:327–335. Retrieved from: https://www.researchgate.net/publication/43202429_Chemical_composition_and_larvicidal_evaluation_ of_Mentha_Salvia_and_Melissa_essential_oils_against_the_West_Nile_virus_mosquito_Culex_pipiens
32. Leyva-López N, Gutiérrez-Grijalva EP, Vazquez-Olivo G, Heredia JB. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules. 2017; 22(6):989. https://doi.org/10.3390/molecules22060989
33. Teixeira, B., Marques, A., Ramos, C., Serrano, C., Matos, O., Neng, N. R., Nogueira, J. M., Saraiva, J. A., & Nunes, M. L. (2013). Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. Journal of the science of food and agriculture, 93(11), 2707–2714. Retrieved from https://www.researchgate.net/publication/236104725_Chemical_composition_and_bioactivity_of_different_ oregano_Origanum_vulgare_extracts_and_essential_oil
34. Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. https://doi.org/10.3390/ijms21249653
35. Teixeira, B., Marques, A., Ramos, C., Serrano, C., Matos, O., Neng, N. R., Nogueira, J. M., Saraiva, J. A., & Nunes, M. L. (2013). Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. Journal of the science of food and agriculture, 93(11), 2707–2714. Retrieved from https://www.researchgate.net/publication/236104725_Chemical_composition_and_bioactivity_of_different_ oregano_Origanum_vulgare_extracts_and_essential_oil
36. Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. https://doi.org/10.1016/j.phytochem.2015.09.008
37. Herrera-Rodríguez, S.E.; López-Rivera, R.J.; García-Márquez, E.; Estarrón-Espinosa, M.; Espinosa-Andrews, H. Mexican oregano (Lippia graveolens) essential oil-in-water emulsions: Impact of emulsifier type on the antifungal activity of Candida albicans. Food Sci. Biotechnol. 2019, 28, 441–448. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6431336/
38. Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. https://doi.org/10.3390/molecules26175156
39. Petropoulos, S. A., Daferera, D., Polissiou, M. G., & Passam, H. C. (2010). Effect of freezing, drying and the duration of storage on the composition of essential oils of plain-leafed parsley [Petroselinum crispum (Mill.) Nym. ssp. neapolitanum Danert] and turnip-rooted parsley [Petroselinum crispum (Mill.) Nym. ssp. tuberosum (Bernh.) Crov.]. Flavour and fragrance journal, 25(1), 28-34. Retrieved from: https://www. academia.edu/11743067/A_8_%CE%95ffect_of_freezing_ drying_and_the_duration_of_storage_on_the_essential_oil_composition_of_plain_leafed_parsley_Petroselinum_crispum_%CE%9Cill_%CE%9Dym_ssp_neapolitanum_Danert_and_turnip_rooted_parsley_Petroselinum_ crispum_Mill_Nym_ssp_tuberosum_Bernh_Crov_
40. Farouk, A., Ali, H., Al-Khalifa, A. R., Mohsen, M., & Fikry, R. (2017). Aroma volatile compounds of parsley cultivated in the Kingdom of Saudi Arabia and Egypt extracted by hydrodistillation and headspace solid-phase microextraction. International journal of food properties, 20(sup3), S2868-S2877. https://doi.org/10.1 080/10942912.2017.1381707
41. Masanetz, C., & Grosch, W. (1998). Key odorants of parsley leaves (Petroselinum crispum [Mill.] Nym. ssp. crispum) by Odour–activity values. Flavour and fragrance journal, 13(2), 115-124. https://doi.org/10.1002/ (SICI)1099-1026(199803/04)13:2<115::AID-FFJ706>3.0.CO;2-6
42. MacLeod, A. J.; Snyder, C. H.; Subramanian, G. Volatile Aroma Constituents of Parsley Leaves. Photochemistry. 1985, 24, 2623–2627. www.sciencedirect.com/science/article/abs/pii/S0031942200806822
43. Aziz E.E., Sabry R.M., Ahmed S.S. (2013). Plant Growth and Essential Oil Production of Sage (Salvia officinalis L.) and Curly- Leafed Parsley (Petroselinum crispum ssp. crispum L.) Cultivated under Salt Stress Conditions. World Appl. Sci. J. 2013;28:785– 796. Retrieved from https://www.researchgate.net/profile/ Eman-Aziz/publication/286537403_Plant_growth_and_ essential_oil_production_of_sage_Salvia_officinalis_L_ and_curly-leafed_parsley_Petroselinum_crispum_ssp_ crispum_L_cultivated_under_salt_stress_conditions
44. Tschiggerl, C.; Bucar, F. Investigation of the Volatile Fraction of Rosemary Infusion Extracts. Sci. Pharm. 2010, 78, 483-492. https://doi.org/10.3797/scipharm.1004-23
45. Micić, D., Đurović, S., Riabov, P., Tomić, A., Šovljanski, O., Filip, S., Tosti, T., Dojčinović, B., Božović, R., Jovanović, D., & Blagojević, S. (2021). Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity, and Gastronomical Perspectives. Foods (Basel, Switzerland), 10(11), 2734. https://doi.org/10.3390/foods10112734.
46. Karakaya S., El S.N., Karagozlu N., Sahin S., Sumnu G., Bayramoglu B. Microwave-assisted hydrodistillation of essential oil from rosemary. J. Food Sci. Technol. 2014;51:1056–1065. doi: 10.1007/s13197-011-0610-y. https:// www.ncbi.nlm.nih.gov/pmc/articles/PMC4033739/
47. Hamidpour, M., Hamidpour, R., Hamidpour, S., & Shahlari, M. (2014). Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. Journal of traditional and complementary medicine, 4(2), 82–88. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4003706/
48. Jonas, M., & Schieberle, P. (2021). Characterization of the key aroma compounds in fresh leaves of garden sage (Salvia officinalis L.) by means of the sensomics approach: influence of drying and storage and comparison with commercial dried sage. Journal of Agricultural and Food Chemistry, 69(17), 5113-5124. https://pubs.acs.org/doi/10.1021/acs.jafc.1c01275
49. Bassoli, A., Borgonovo, G., Tosca, A., Spoleto, P., Martinetti, L., & Ferrante, A. (2010, August). Characterization of some qualitative traits in different perilla cultivars. In XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 939 (pp. 301-308). Retrieved from: https://www.researchgate.net/publication/259888321_Characterization_of_some_qualitative_traits_in_different_perilla_cultivars 50. Nitta, M., Kobayashi, H., Ohnishi-Kameyama, M., Nagamine, T., & Yoshida, M. (2006). Essential oil variation of cultivated and wild Perilla analyzed by GC/ MS. Biochemical Systematics and Ecology, 34(1), 25-37.
51. Ito, M. (2008). Studies on perilla relating to its essential oil and taxonomy. Phytochemistry research progress. www.google. com/books/edition/Phytochemistry_Research_Progress/l5 Mo0M5CqQcC?hl=en&gbpv=1&pg=PA13&printsec=frontcover
52. Ekiert, H., Świątkowska, J., Knut, E., Klin, P., Rzepiela, A., Tomczyk, M., & Szopa, A. (2021). Artemisia dracunculus (Tarragon): A review of its traditional uses, phytochemistry and pharmacology. Frontiers in Pharmacology, 12, 653993. https://doi.org/10.3389/fphar.2021.653993
53. Sonmezdag, A. S., Kelebek, H., & Selli, S. (2016). Characterization of aroma-active and phenolic profiles of wild thyme (Thymus serpyllum) by GC-MS-Olfactometry and LC-ESI-MS/MS. Journal of food science and technology, 53(4), 1957–1965. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4926906/
54. Hudaib, M., & Aburjai, T. (2007). Volatile components of Thymus vulgaris L. from wild-growing and cultivated plants in Jordan. Flavour and fragrance journal, 22(4), 322-327. Retrieved from www.researchgate.net/profile/Talal-Aburjai/publication/227753455_Volatile_components_of_Thymus_ vulgaris_L_from_wild-growing_and_cultivated_ plants_in_Jordan/links/5a2fd3430f7e9b0d50f6fe12/ Volatile-components-of-Thymus-vulgaris-L-from- wild-growing-and-cultivated-plants-in-Jordan.pdf
55. Calín-Sánchez, Á., Figiel, A., Lech, K., Szumny, A., & Carbonell- Barrachina, Á. A. (2013). Effects of drying methods on the composition of thyme (Thymus vulgaris L.) essential oil. Drying technology, 31(2), 224-235. Retrieved from: https://www.researchgate.net/publication/235736520_ Effects_of_Drying_Methods_on_the_Composition_ of_Thyme_Thymus_vulgaris_L_Essential_Oil
56. Lee, S. J., Umano, K., Shibamoto, T., & Lee, K. G. (2005). Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food chemistry, 91(1), 131-137. https://www.researchgate.net/publication/238378687_ Identification_of_volatile_components_in_basil_L_ and_thyme_leaves_L_and_their_antioxidant_properties
57. Jurevičiūtė, R., Ložienė, K., Bruno, M., Maggio, A., & Rosselli, S. (2019). Composition of essential oil of lemon thyme (Thymus× citriodorus) at different hydrodistillation times. Natural product research, 33(1), 80-88. Retrieved from: https://www.researchgate.net/profile/Kristina-Loziene/ publication/322915167_Composition_of_essential_oil_of_lemon_thyme_Thymus_citriodorus_at_different_ hydrodistillation_times/links/61372bcf2b40ec7d8bed92e9/ Composition-of-essential-oil-of-lemon-thyme-Thymus- citriodorus-at-different-hydrodistillation-times.pdf
2. De Martino L, Caputo L, Amato G, Iannone M, Barba AA, De Feo V. Postharvest Microwave Drying of Basil (Ocimum basilicum L.): The Influence of Treatments on the Quality of Dried Products. Foods. 2022; 11(7):1029. https://doi.org/10.3390/foods11071029
3. Lee, S. J., Umano, K., Shibamoto, T., & Lee, K. G. (2005). Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food chemistry, 91(1), 131-137. https://www.researchgate.net/publication/238378687_Identification_of_volatile_components_in_basil_L_ and_thyme_leaves_L_and_their_antioxidant_properties
4. Tangpao T, Chung H-H, Sommano SR. Aromatic Profiles of Essential Oils from Five Commonly Used Thai Basils. Foods. 2018; 7(11):175. https://doi.org/10.3390/foods7110175 5. Sellami, I. H., Wannes, W. A., Bettaieb, I., Berrima, S., Chahed, T., Marzouk, B., & Limam, F. (2011). Qualitative and quantitative changes in the essential oil of Laurus nobilis L. leaves as affected by different drying methods. Food chemistry, 126(2), 691-69. https://www.sciencedirect. com/science/article/abs/pii/S0308814610014299
6. Kilic, A., Hafizoglu, H., Kollmannsberger, H., & Nitz, S. (2004). Volatile constituents and key odorants in leaves, buds, flowers, and fruits of Laurus nobilis L. Journal of agricultural and food chemistry, 52(6), 1601-1606. Retrieved from www.academia.edu/741769/Volatile_constituents_and_key_odorants_in_ leaves_buds_flowers_and_fruits_of_Laurus_nobilis_L
7. Wikipedia contributors. (2023, May 23). Bay leaf. In Wikipedia, The Free Encyclopedia. Retrieved 10:39, June 17, 2023, from https://en.wikipedia. org/w/index.php?title=Bay_leaf&oldid=1156462697
8. Mandal, S., & Mandal, M. (2015). Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine, 5(6), 421-428. https://www. sciencedirect.com/science/article/pii/S2221169115000647
9. Łyczko, J.; Masztalerz, K.; Lipan, L.; Iwiński, H.; Lech, K.; Carbonell- Barrachina, Á.A.; Szumny, A. Coriandrum sativum L.—Effect of Multiple Drying Techniques on Volatile and Sensory Profile. Foods 2021, 10, 403. https://doi.org/10.3390/foods10020403
10. Shahwar, M. K., El-Ghorab, A. H., Anjum, F. M., Butt, M. S., Hussain, S., & Nadeem, M. (2012). Characterization of coriander (Coriandrum sativum L.) seeds and leaves: volatile and non volatile extracts. International journal of food properties, 15(4), 736-747. https://doi.org/10.1080/10942912.2010.500068
11. Mandal, S., & Mandal, M. (2015). Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine, 5(6), 421-428. https://www.sciencedirect.com/science/article/pii/S2221169115000647
12. Bhuiyan, M. N. I., Begum, J., & Sultana, M. (2009). Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. ||| Bangladesh Journal of Pharmacology|||, 4(2), 150-153. http://bdpsjournal.org/index.php/bjp/article/view/395
13. Kumar S, Ahmad R, Saeed S, Azeem M, Mozūraitis R, Borg-Karlson A-K and Zhu G (2022) Chemical Composition of Fresh Leaves Headspace Aroma and Essential Oils of Four Coriander Cultivars. Front. Plant Sci. 13:820644. https://doi.org/10.3389/fpls.2022.820644
14. Jana, S., & Shekhawat, G. S. (2010). Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacognosy reviews, 4(8), 179–184. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3249919/
15. Jensen, P., Tielkemeier, S., & Zeigler, N. (2020, May). Yakima Chief Hops: Getting the Most from your Hops. Brewers Association. https://www.brewersassociation. org/wp-content/uploads/2020/05/CBC-Online- Seminar-Presentation-Getting-the-Most-from- Your-Hops-Presented-by-Yakima-Chief-Hops.pdf
16. Kemp, O., Hofmann, S., Braumann, I., Jensen, S., Fenton, A., & Oladokun, O. (2021). Changes in key hop-derived compounds and their impact on perceived dry-hop flavour in beers after storage at cold and ambient temperature. Journal of the Institute of Brewing, 127(4), 367-384. https://onlinelibrary.wiley.com/doi/full/10.1002/jib.667
17. Hieronymus, S. (2016, March 16). Hops Oils & Aroma: Uncharted Waters. Craft Beer & Brewing. Retrieved June 28, 2023, from https:// beerandbrewing.com/hops-oils--aroma-uncharted-waters/
18. Mukarram M, Choudhary S, Khan MA, Poltronieri P, Khan MMA, Ali J, Kurjak D, Shahid M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants. 2022; 11(1):20. https://doi.org/10.3390/antiox11010020
19. Meena, S.; Kumar, S.R.; Rao, D.K.V.; Dwivedi, V.; Shilpashree, H.B.; Rastogi, S.; Shasany, A.K.; Nagegowda, D.A. De Novo sequencing and analysis of lemongrass transcriptome provide first insights into the essential oil biosynthesis of aromatic grasses. Front. Plant Sci. 2016, 7, 1129. https://doi.org/10.3389/fpls.2016.01129
20. Saleem M, Afza N, Anwar MA, Hai SM, Ali MS. Comparative study of essential oils of Cymbopogon citratus and some members of the Genus Citrus. Nat Prod Res. 2003;17:369–73. Retrieved from: https://www.researchgate.net/publication/9065118_A_Comparative_Study_of_Essential_Oils_of_Cymbopogon_ Citratus_and_Some_Members_of_the_Genus_Citrus
21. Chanthai, S., Prachakoll, S., Ruangviriyachai, C., & Luthria, D. L. (2012). Influence of extraction methodologies on the analysis of five major volatile aromatic compounds of citronella grass (Cymbopogon nardus) and lemongrass (Cymbopogon citratus) grown in Thailand. Journal of AOAC International, 95(3), 763-772. https://doi.org/10.5740/jaoacint.11-335
22. Park, Y. J., Baskar, T. B., Yeo, S. K., Arasu, M. V., Al-Dhabi, N. A., Lim, S. S., & Park, S. U. (2016). Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus, 5, 1-10. https://doi.org/10.1186/s40064-016-3283-1
23. Vining, K. J., Hummer, K. E., Bassil, N. V., Lange, B. M., Khoury, C. K., & Carver, D. (2020). Crop wild relatives as germplasm resource for cultivar improvement in mint (Mentha L.). Frontiers in Plant Science, 11, 1217. https:// www.frontiersin.org/articles/10.3389/fpls.2020.01217/full
24. Srivastava, R. K., Singh, A. K., Kalra, A., Tomar, V. K. S., Bansal, R. P., Patra, D. D., ... & Kumar, S. (2002). Characteristics of menthol mint Mentha arvensis cultivated on industrial scale in the Indo- Gangetic plains. Industrial crops and products, 15(3), 189-198.
25. Peters, V. C. T., Dunkel, A., Frank, O., McCormack, B., Dowd, E., Didzbalis, J., ... & Hofmann, T. (2021). A high throughput toolbox for comprehensive flavor compound mapping in mint. Food Chemistry, 365, 130522. https://doi.org/10.1016/j.foodchem.2021.130522.
26. Buleandra, M., Oprea, E., Popa, D. E., David, I. G., Moldovan, Z., Mihai, I., & Badea, I. A. (2016). Comparative chemical analysis of Mentha piperita and M. spicata and a fast assessment of commercial peppermint teas. Natural product communications, 11(4), 1934578X1601100433. https:// journals.sagepub.com/doi/pdf/10.1177/1934578X1601100433
27. Sun, Z., Wang, H., Wang, J., Zhou, L., & Yang, P. (2014). Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PloS one, 9(12), e114767. https://journals. plos.org/plosone/article?id=10.1371/journal.pone.0114767
28. Schmidt, E., Bail, S., Buchbauer, G., Stoilova, I., Atanasova, T., Stoyanova, A., ... & Jirovetz, L. (2009). Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha x piperita. Natural product communications, 4(8). https:// journals.sagepub.com/doi/pdf/10.1177/1934578X0900400819
29. Soković MD, Vukojević J, Marin PD, Brkić DD, Vajs V, Van Griensven LJ (2009) Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 14:238–249. https://doi.org/10.3390/molecules14010238
30. Kelley, L. E., & Cadwallader, K. R. (2017). Identification and quantitation of potent odorants in spearmint oils. Journal of agricultural and food chemistry, 66(10), 2414- 2421. https://pubs.acs.org/doi/abs/10.1021/acs.jafc.6b04852
31. Koliopoulos G, Pitarokili D, Kioulos E, Michaelakis A, Tzakou O (2010) Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the west nile virus mosquito Culex pipiens. Parasitol Res 107:327–335. Retrieved from: https://www.researchgate.net/publication/43202429_Chemical_composition_and_larvicidal_evaluation_ of_Mentha_Salvia_and_Melissa_essential_oils_against_the_West_Nile_virus_mosquito_Culex_pipiens
32. Leyva-López N, Gutiérrez-Grijalva EP, Vazquez-Olivo G, Heredia JB. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules. 2017; 22(6):989. https://doi.org/10.3390/molecules22060989
33. Teixeira, B., Marques, A., Ramos, C., Serrano, C., Matos, O., Neng, N. R., Nogueira, J. M., Saraiva, J. A., & Nunes, M. L. (2013). Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. Journal of the science of food and agriculture, 93(11), 2707–2714. Retrieved from https://www.researchgate.net/publication/236104725_Chemical_composition_and_bioactivity_of_different_ oregano_Origanum_vulgare_extracts_and_essential_oil
34. Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. https://doi.org/10.3390/ijms21249653
35. Teixeira, B., Marques, A., Ramos, C., Serrano, C., Matos, O., Neng, N. R., Nogueira, J. M., Saraiva, J. A., & Nunes, M. L. (2013). Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. Journal of the science of food and agriculture, 93(11), 2707–2714. Retrieved from https://www.researchgate.net/publication/236104725_Chemical_composition_and_bioactivity_of_different_ oregano_Origanum_vulgare_extracts_and_essential_oil
36. Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. https://doi.org/10.1016/j.phytochem.2015.09.008
37. Herrera-Rodríguez, S.E.; López-Rivera, R.J.; García-Márquez, E.; Estarrón-Espinosa, M.; Espinosa-Andrews, H. Mexican oregano (Lippia graveolens) essential oil-in-water emulsions: Impact of emulsifier type on the antifungal activity of Candida albicans. Food Sci. Biotechnol. 2019, 28, 441–448. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6431336/
38. Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. https://doi.org/10.3390/molecules26175156
39. Petropoulos, S. A., Daferera, D., Polissiou, M. G., & Passam, H. C. (2010). Effect of freezing, drying and the duration of storage on the composition of essential oils of plain-leafed parsley [Petroselinum crispum (Mill.) Nym. ssp. neapolitanum Danert] and turnip-rooted parsley [Petroselinum crispum (Mill.) Nym. ssp. tuberosum (Bernh.) Crov.]. Flavour and fragrance journal, 25(1), 28-34. Retrieved from: https://www. academia.edu/11743067/A_8_%CE%95ffect_of_freezing_ drying_and_the_duration_of_storage_on_the_essential_oil_composition_of_plain_leafed_parsley_Petroselinum_crispum_%CE%9Cill_%CE%9Dym_ssp_neapolitanum_Danert_and_turnip_rooted_parsley_Petroselinum_ crispum_Mill_Nym_ssp_tuberosum_Bernh_Crov_
40. Farouk, A., Ali, H., Al-Khalifa, A. R., Mohsen, M., & Fikry, R. (2017). Aroma volatile compounds of parsley cultivated in the Kingdom of Saudi Arabia and Egypt extracted by hydrodistillation and headspace solid-phase microextraction. International journal of food properties, 20(sup3), S2868-S2877. https://doi.org/10.1 080/10942912.2017.1381707
41. Masanetz, C., & Grosch, W. (1998). Key odorants of parsley leaves (Petroselinum crispum [Mill.] Nym. ssp. crispum) by Odour–activity values. Flavour and fragrance journal, 13(2), 115-124. https://doi.org/10.1002/ (SICI)1099-1026(199803/04)13:2<115::AID-FFJ706>3.0.CO;2-6
42. MacLeod, A. J.; Snyder, C. H.; Subramanian, G. Volatile Aroma Constituents of Parsley Leaves. Photochemistry. 1985, 24, 2623–2627. www.sciencedirect.com/science/article/abs/pii/S0031942200806822
43. Aziz E.E., Sabry R.M., Ahmed S.S. (2013). Plant Growth and Essential Oil Production of Sage (Salvia officinalis L.) and Curly- Leafed Parsley (Petroselinum crispum ssp. crispum L.) Cultivated under Salt Stress Conditions. World Appl. Sci. J. 2013;28:785– 796. Retrieved from https://www.researchgate.net/profile/ Eman-Aziz/publication/286537403_Plant_growth_and_ essential_oil_production_of_sage_Salvia_officinalis_L_ and_curly-leafed_parsley_Petroselinum_crispum_ssp_ crispum_L_cultivated_under_salt_stress_conditions
44. Tschiggerl, C.; Bucar, F. Investigation of the Volatile Fraction of Rosemary Infusion Extracts. Sci. Pharm. 2010, 78, 483-492. https://doi.org/10.3797/scipharm.1004-23
45. Micić, D., Đurović, S., Riabov, P., Tomić, A., Šovljanski, O., Filip, S., Tosti, T., Dojčinović, B., Božović, R., Jovanović, D., & Blagojević, S. (2021). Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity, and Gastronomical Perspectives. Foods (Basel, Switzerland), 10(11), 2734. https://doi.org/10.3390/foods10112734.
46. Karakaya S., El S.N., Karagozlu N., Sahin S., Sumnu G., Bayramoglu B. Microwave-assisted hydrodistillation of essential oil from rosemary. J. Food Sci. Technol. 2014;51:1056–1065. doi: 10.1007/s13197-011-0610-y. https:// www.ncbi.nlm.nih.gov/pmc/articles/PMC4033739/
47. Hamidpour, M., Hamidpour, R., Hamidpour, S., & Shahlari, M. (2014). Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. Journal of traditional and complementary medicine, 4(2), 82–88. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4003706/
48. Jonas, M., & Schieberle, P. (2021). Characterization of the key aroma compounds in fresh leaves of garden sage (Salvia officinalis L.) by means of the sensomics approach: influence of drying and storage and comparison with commercial dried sage. Journal of Agricultural and Food Chemistry, 69(17), 5113-5124. https://pubs.acs.org/doi/10.1021/acs.jafc.1c01275
49. Bassoli, A., Borgonovo, G., Tosca, A., Spoleto, P., Martinetti, L., & Ferrante, A. (2010, August). Characterization of some qualitative traits in different perilla cultivars. In XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 939 (pp. 301-308). Retrieved from: https://www.researchgate.net/publication/259888321_Characterization_of_some_qualitative_traits_in_different_perilla_cultivars 50. Nitta, M., Kobayashi, H., Ohnishi-Kameyama, M., Nagamine, T., & Yoshida, M. (2006). Essential oil variation of cultivated and wild Perilla analyzed by GC/ MS. Biochemical Systematics and Ecology, 34(1), 25-37.
51. Ito, M. (2008). Studies on perilla relating to its essential oil and taxonomy. Phytochemistry research progress. www.google. com/books/edition/Phytochemistry_Research_Progress/l5 Mo0M5CqQcC?hl=en&gbpv=1&pg=PA13&printsec=frontcover
52. Ekiert, H., Świątkowska, J., Knut, E., Klin, P., Rzepiela, A., Tomczyk, M., & Szopa, A. (2021). Artemisia dracunculus (Tarragon): A review of its traditional uses, phytochemistry and pharmacology. Frontiers in Pharmacology, 12, 653993. https://doi.org/10.3389/fphar.2021.653993
53. Sonmezdag, A. S., Kelebek, H., & Selli, S. (2016). Characterization of aroma-active and phenolic profiles of wild thyme (Thymus serpyllum) by GC-MS-Olfactometry and LC-ESI-MS/MS. Journal of food science and technology, 53(4), 1957–1965. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4926906/
54. Hudaib, M., & Aburjai, T. (2007). Volatile components of Thymus vulgaris L. from wild-growing and cultivated plants in Jordan. Flavour and fragrance journal, 22(4), 322-327. Retrieved from www.researchgate.net/profile/Talal-Aburjai/publication/227753455_Volatile_components_of_Thymus_ vulgaris_L_from_wild-growing_and_cultivated_ plants_in_Jordan/links/5a2fd3430f7e9b0d50f6fe12/ Volatile-components-of-Thymus-vulgaris-L-from- wild-growing-and-cultivated-plants-in-Jordan.pdf
55. Calín-Sánchez, Á., Figiel, A., Lech, K., Szumny, A., & Carbonell- Barrachina, Á. A. (2013). Effects of drying methods on the composition of thyme (Thymus vulgaris L.) essential oil. Drying technology, 31(2), 224-235. Retrieved from: https://www.researchgate.net/publication/235736520_ Effects_of_Drying_Methods_on_the_Composition_ of_Thyme_Thymus_vulgaris_L_Essential_Oil
56. Lee, S. J., Umano, K., Shibamoto, T., & Lee, K. G. (2005). Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food chemistry, 91(1), 131-137. https://www.researchgate.net/publication/238378687_ Identification_of_volatile_components_in_basil_L_ and_thyme_leaves_L_and_their_antioxidant_properties
57. Jurevičiūtė, R., Ložienė, K., Bruno, M., Maggio, A., & Rosselli, S. (2019). Composition of essential oil of lemon thyme (Thymus× citriodorus) at different hydrodistillation times. Natural product research, 33(1), 80-88. Retrieved from: https://www.researchgate.net/profile/Kristina-Loziene/ publication/322915167_Composition_of_essential_oil_of_lemon_thyme_Thymus_citriodorus_at_different_ hydrodistillation_times/links/61372bcf2b40ec7d8bed92e9/ Composition-of-essential-oil-of-lemon-thyme-Thymus- citriodorus-at-different-hydrodistillation-times.pdf