Hawai'i Beverage Guides Libation Marvels: Stills, Production and Distillation
A Guide to: Distillation
Distillation is the process of separating and removing alcohol from the fermented liquid of agricultural raw ingredients. This technology has changed over the centuries as the understanding of physics and chemistry has improved.
|
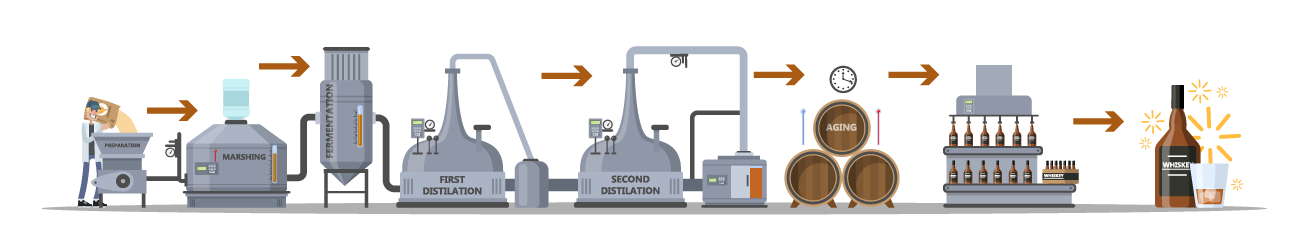
General Distillation Process
1. ETHANOL FERMENTATION is the process of converting glucose, fructose and sucrose into ethanol and carbon dioxide. However, beyond an alcoholic content of 4-12% ABV, the fermented liquid also contains water, other chemical byproducts including esters and other volatile (flavor) compounds, and unwanted solids. A distillate is only as good as the fermentation, because distillation is the process of refining and concentrating (not changing) what was already made. In other words, distillation typically does not add anything; it only removes the unwanted parts. 2. Fractional distillation is the process of separating an azeotrope mixture (a combination of two or more liquids whose proportions cannot be altered or changed by simple distillation) into different components by the application of heat. This results in separate compounds by differences in boiling point, and through the creation of reflux. • The application of heat separates various compounds within the fermented liquid due to the differences in boiling points. Ethanol in an azeotrope mixture with 4% water boils at approximately 173°F, while water boils at 212°F. • Reflux is the process of condensing vapors back into liquid so that the liquid can be (re)vaporized. As liquids with similar boiling points typically do not separate on their first cycle of refluxing, additional reflux allows for more congeners to be removed. Combined with the increased amount of copper contact, the more reflux there is, the more pure the ethanol produced. In other words, the more reflux, the “lighter” the spirit. |
Reflux ratio: The ratio of the liquid that has returned to the distillation column to the amount of liquid removed.
Forced Reflux: Reflux can be forced by using cooling tubes running through the column, a water jacket running around the column, or a dephlegmator to condense the vapors. The speed of distillation, and therefore the reflux ratio, is controlled by the speed and temperature of the cooling water being run through the lines. 3. Chemical reaction with copper • Copper removes undesirable sulfurs from the distillate, reduces bacterial contamination, and can prevent the production of ethyl carbamate, a toxic substance formed from cyanides found in the seeds of fruits. Sulfur in particular is the compound of most concern, and the more the mixture interacts with copper, the more these sulfurs get stripped out. • For more on the importance of copper and where it must be located in a still to impact distillation, read: Harrison, Barry & Fagnen, Olivier & Jack, Frances & Brosnan, James. (2011). The Impact of Copper in Different Parts of Malt Whisky Pot Stills on New Make Spirit Composition and Aroma. Journal- Institute of Brewing. 117. 106-122. 10.1002/j.2050- 0416.2011.tb00450.x. https://onlinelibrary.wiley.com/doi/ pdf/10.1002/j.2050-0416.2011.tb00450.x • Freeze distillation, which is not typically used in the production of ethanol, is the process of separating a liquid by differences in freezing point. To produce ethanol in a freeze distillation, the mixture is chilled, and due to the fact that alcohol has a colder freezing point than water, the water will solidify and the liquid ethanol is extracted. |
Some products claim to be over 3 times distilled, yet this can be somewhat misleading. According to the 27 CFR § 5.42 (b)(6) - Prohibited Practices: “Distilled spirits may not be labeled as “double distilled” or “triple distilled” or any similar term unless it is a truthful statement of fact. For purposes of this paragraph only, a distillation means a single run through a pot still or a single run through a column of a column (reflux) still. The number of distillations may be understated but may not be overstated.” Commonly whiskey, tequila and some other spirits are double distilled in a pot still, which means they have run through twice. When it comes to vodka, products marked “ over three times distilled” are highly variable in their claims as to what they call a distillation cycle, and will sometimes refer to the number of plates in a distillation column as they are “counting” the number of times the spirits has vaporized and condensed. It may also refer to the number of columns in the distillation run.
Separation by boiling point (Cuts) 5, 6, 7
After reflux has helped to separate out the different liquids from the azeotropic mixture, they need to be collected. This collection is done by varying the temperature of the distillation run to group compounds by boiling point. Though many of these compounds are in the final distillate, the distiller is selecting for quantity of the compound. In distillation, these groupings are called:
Foreshots Heads Hearts Tails
Composed of
Acetone with a boiling point of 133°F (56 °C).
Acetaldehyde with a boiling point of 69°F (20.55°C)
Methanol (wood alcohol) with a boiling point of 147°F (64°C) Acetone, Methanol
Ethyl-acetate with a boiling point of 171°F (77.1°C ) and some Ethanol.
Predominantly Ethanol: 173°F (78°C) Ethanol and Fusel oils:
1-Propanol a boiling point of 207°F (97.2°C)
Butanol alcohol has a boiling point of 243°F (117 °C)
Acetic Acid: Acetic Acid has a boiling point of 244°F (117.9 °C)
Furfural has a boiling point of 323°F (161.7 °C)
Vapor Temperature
122°F (50°C) to 175°F (80°C) 175°F (80°C) to 196°F (91°C) 196°F (91°C) to 203°F (95°C) 203°F (95°C) to 208°F (98°C)
ABV NA 80% and above 80% - 60% or 80%-40% 60% or 40% and below
Usage
Foreshots are discarded The heads may be added to the next distillation run, as they may contain a large amount of ethanol. Some can also be blended back into the hearts portion. This is the main part of the distillation run. Tails can be redistilled in a faints run, as tails can also contain a large amount of ethanol and desirable flavors. A portion can also be blended back into the hearts portion.
Feints
The heads and tails that are set aside are collectively referred to as the “feints.” These may be added into the wash on the next run, can be distilled by themselves, or blended back into the hearts portion.
After reflux has helped to separate out the different liquids from the azeotropic mixture, they need to be collected. This collection is done by varying the temperature of the distillation run to group compounds by boiling point. Though many of these compounds are in the final distillate, the distiller is selecting for quantity of the compound. In distillation, these groupings are called:
Foreshots Heads Hearts Tails
Composed of
Acetone with a boiling point of 133°F (56 °C).
Acetaldehyde with a boiling point of 69°F (20.55°C)
Methanol (wood alcohol) with a boiling point of 147°F (64°C) Acetone, Methanol
Ethyl-acetate with a boiling point of 171°F (77.1°C ) and some Ethanol.
Predominantly Ethanol: 173°F (78°C) Ethanol and Fusel oils:
1-Propanol a boiling point of 207°F (97.2°C)
Butanol alcohol has a boiling point of 243°F (117 °C)
Acetic Acid: Acetic Acid has a boiling point of 244°F (117.9 °C)
Furfural has a boiling point of 323°F (161.7 °C)
Vapor Temperature
122°F (50°C) to 175°F (80°C) 175°F (80°C) to 196°F (91°C) 196°F (91°C) to 203°F (95°C) 203°F (95°C) to 208°F (98°C)
ABV NA 80% and above 80% - 60% or 80%-40% 60% or 40% and below
Usage
Foreshots are discarded The heads may be added to the next distillation run, as they may contain a large amount of ethanol. Some can also be blended back into the hearts portion. This is the main part of the distillation run. Tails can be redistilled in a faints run, as tails can also contain a large amount of ethanol and desirable flavors. A portion can also be blended back into the hearts portion.
Feints
The heads and tails that are set aside are collectively referred to as the “feints.” These may be added into the wash on the next run, can be distilled by themselves, or blended back into the hearts portion.
|
Types of Distillation methods
Batch distillation is the traditional process of separating alcohol by time in a pot still. That is, in the distillation of each batch, all the liquid will come out of the same spout, at different times, in order of proof/ABV (Alcohol By Volume). Batch distillation is limited by the capacity of the still. Continuous Distillation in fractionating columns separates alcohol by the location on the still where the alcohol is removed. It should be noted that hybrid stills refer to a batch system that has both a pot and a column. However, these are not continuous distillation systems. |
Clarification: Fractionating Column vs Fractional Distillation
Fractionating Columns (noun) refer to the physical continuous column still. Fractional distillation (verb) refers to the process of separate compounds by differences in their boiling points, and is the process used in both batch and continuous distillation. |
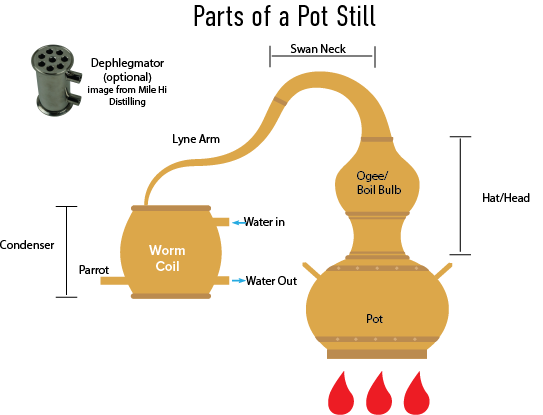
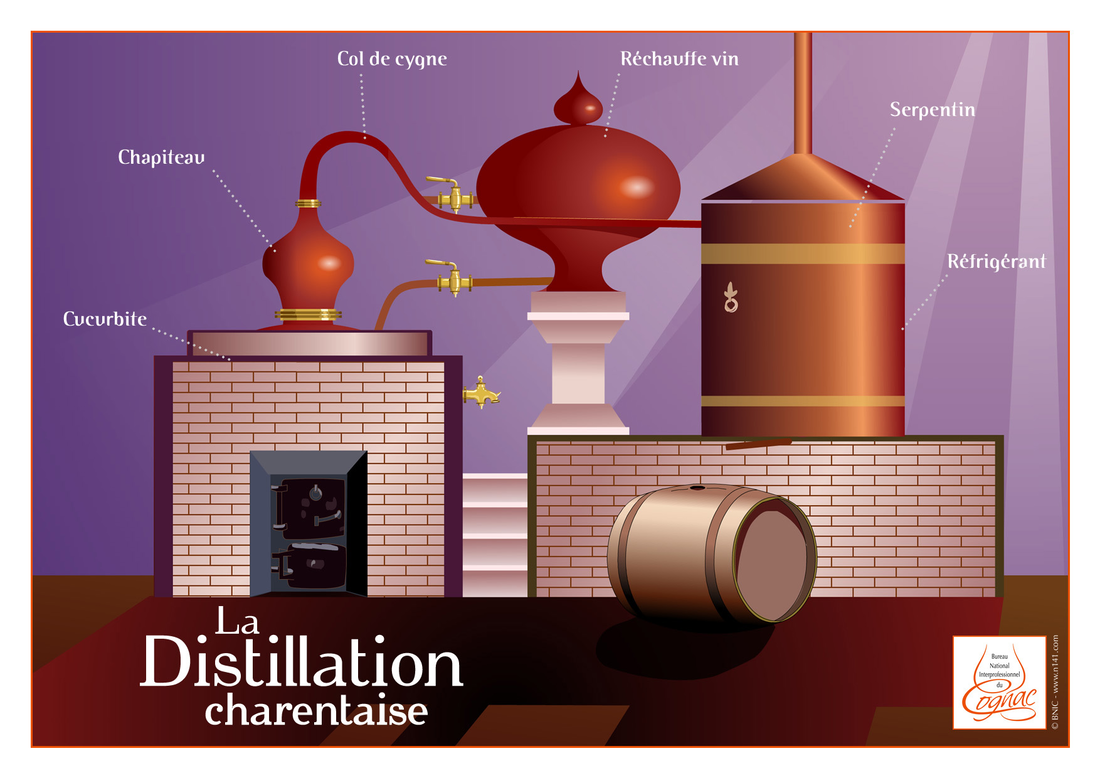
Parts of a Still
Pot: Holds the wash
Head/hat: Used to promote reflux. Different shapes create different amounts of reflux.
Ogee (Optional): A bubble-shaped chamber which allows the distillate to expand, condense, and fall back into the pot during distillation. For more on the history of the ogee read: https://www.edinburghwhiskyacademy.com/blog/ogee-or-not-ogee-that-is-the-question
Boil Bulbs (optional): Provide a place for the rising vapours to condense, thereby promoting reflux.
Swan neck: Connected to the pot via the hat/head, and comes in different shapes and sizes. Most pot stills have a tapered swan neck, allowing for more reflux.
Lyne arm: Sits on top of the swan neck and can be tilted up or down, or can be tapered or straight.
Lyne Angle: The lyne angle is the angle of the lyne arm. If the lyne arm is angled upwards it can be used to create more reflux whereas if it is angled downward the lyne arm has less reflux.
Dephlegmator: The dephlegmator forces reflux by passing the distilled vapor through tubes which are surrounded by water to create a large cooling surface area which condenses some of the distillate and creates reflux.
Worm Coil: The distilled vapor passes through the worm coil to be condensed, into liquid. The coil is typically placed in water or another coolant.
Spirit safe (optional): This glass box with a lock encloses the parrot on Scotch whisky stills. Historically, the safe was only accessible by the local excise tax collector, though now it is primarily decorative. The exterior of the spirit safe has controls that allow the distiller to make cuts by directing the foreshots, heads, hearts, and tails to various receivers.
Parrot: Where the final distillate leaves the still
Gin Basket (Optional)
Gin baskets are perforated metal containers that the botanicals for gin are placed into. The gin basket is then placed into the vapor path.
Copper "catalysts" inside the stainless steel pot, placed in the path of alcohol-water vapor can be used instead of making the entire pot out of copper. This is because the catalyst can create higher amounts of surface area than the pot. The copper catalyst can also use less copper than the pot, which lowers the price of the distillation system. For some insight into copper catalysts, visit: a-holstein.de/en/distilleries/options/copper-catalyst/
Head/hat: Used to promote reflux. Different shapes create different amounts of reflux.
Ogee (Optional): A bubble-shaped chamber which allows the distillate to expand, condense, and fall back into the pot during distillation. For more on the history of the ogee read: https://www.edinburghwhiskyacademy.com/blog/ogee-or-not-ogee-that-is-the-question
Boil Bulbs (optional): Provide a place for the rising vapours to condense, thereby promoting reflux.
Swan neck: Connected to the pot via the hat/head, and comes in different shapes and sizes. Most pot stills have a tapered swan neck, allowing for more reflux.
Lyne arm: Sits on top of the swan neck and can be tilted up or down, or can be tapered or straight.
Lyne Angle: The lyne angle is the angle of the lyne arm. If the lyne arm is angled upwards it can be used to create more reflux whereas if it is angled downward the lyne arm has less reflux.
Dephlegmator: The dephlegmator forces reflux by passing the distilled vapor through tubes which are surrounded by water to create a large cooling surface area which condenses some of the distillate and creates reflux.
Worm Coil: The distilled vapor passes through the worm coil to be condensed, into liquid. The coil is typically placed in water or another coolant.
Spirit safe (optional): This glass box with a lock encloses the parrot on Scotch whisky stills. Historically, the safe was only accessible by the local excise tax collector, though now it is primarily decorative. The exterior of the spirit safe has controls that allow the distiller to make cuts by directing the foreshots, heads, hearts, and tails to various receivers.
Parrot: Where the final distillate leaves the still
Gin Basket (Optional)
Gin baskets are perforated metal containers that the botanicals for gin are placed into. The gin basket is then placed into the vapor path.
Copper "catalysts" inside the stainless steel pot, placed in the path of alcohol-water vapor can be used instead of making the entire pot out of copper. This is because the catalyst can create higher amounts of surface area than the pot. The copper catalyst can also use less copper than the pot, which lowers the price of the distillation system. For some insight into copper catalysts, visit: a-holstein.de/en/distilleries/options/copper-catalyst/
-
Still Materials
-
Heating of the Still
-
Still Size and Shape
<
>
Still materials
Wood
Wooden stills are hyper-rare. There are three wooden stills made of greenheart wood at Demerara Distillers in Guyana, used in the production of rum. Wooden stills made of Sugi (Japanese cedar) are historically used to make shochu.
Benefits: Wood is cheap. It’s easy to fix. It can also impart flavor.
Drawbacks: Wood does not heat the mash, so a heating element like steam is required. Wood also is hard to maintain.
Clay
Benefits: Clay pots are easier to produce or procure in remote locations compared to metal or glass. They are more durable than wood, and easier to maintain. They were also convenient when distilling was illegal, because when deconstructed, a clay pot is just a clay pot. They can also be heated directly by fire.
Drawbacks: Clay is brittle. The condenser and the pot do not join completely together, leading to some of the distillation escaping as vapor. Clay also does not heat evenly through conduction.
Glass
Benefits: The theoretical benefit of glass is that it does not impart flavors that may be imparted by stainless steel or copper. Copper, however, is used in portions of the still.
Drawbacks: Glass is fragile, and heats slowly. It can heat unevenly if a direct fire method is used, and is difficult to make in a large size.
Stainless Steel
Benefits: Stainless steel is cheaper than copper, easier to clean, and heats quickly and evenly.
Drawbacks: It does not interact with sulfur like copper does. Stainless steel is more critical to use in areas of the still where reflux occurs, because it interacts with the vapors, and not in the places that serve as a heated container, since very limited reactions between sulfur and copper occur in those places.
Copper
Benefits: Copper is highly conductive, therefore heats quickly and evenly.
Drawbacks: It is more expensive than stainless steel.
The quantity of copper the still is made of influences the amount of Sulfur that is capable of being absorbed. The reaction is: Cu + S = Cu2S; however, when O2 and heat are added to the equation when fresh air is let into the still during distillation: Cu2S + O2 (heated) = Cu + 2S02
Wood
Wooden stills are hyper-rare. There are three wooden stills made of greenheart wood at Demerara Distillers in Guyana, used in the production of rum. Wooden stills made of Sugi (Japanese cedar) are historically used to make shochu.
Benefits: Wood is cheap. It’s easy to fix. It can also impart flavor.
Drawbacks: Wood does not heat the mash, so a heating element like steam is required. Wood also is hard to maintain.
Clay
Benefits: Clay pots are easier to produce or procure in remote locations compared to metal or glass. They are more durable than wood, and easier to maintain. They were also convenient when distilling was illegal, because when deconstructed, a clay pot is just a clay pot. They can also be heated directly by fire.
Drawbacks: Clay is brittle. The condenser and the pot do not join completely together, leading to some of the distillation escaping as vapor. Clay also does not heat evenly through conduction.
Glass
Benefits: The theoretical benefit of glass is that it does not impart flavors that may be imparted by stainless steel or copper. Copper, however, is used in portions of the still.
Drawbacks: Glass is fragile, and heats slowly. It can heat unevenly if a direct fire method is used, and is difficult to make in a large size.
Stainless Steel
Benefits: Stainless steel is cheaper than copper, easier to clean, and heats quickly and evenly.
Drawbacks: It does not interact with sulfur like copper does. Stainless steel is more critical to use in areas of the still where reflux occurs, because it interacts with the vapors, and not in the places that serve as a heated container, since very limited reactions between sulfur and copper occur in those places.
Copper
Benefits: Copper is highly conductive, therefore heats quickly and evenly.
Drawbacks: It is more expensive than stainless steel.
The quantity of copper the still is made of influences the amount of Sulfur that is capable of being absorbed. The reaction is: Cu + S = Cu2S; however, when O2 and heat are added to the equation when fresh air is let into the still during distillation: Cu2S + O2 (heated) = Cu + 2S02
Types of Fuel Used to Heat Stills
Gas vs. Electric is more a matter of cost and potential environmental impact. The choice is often dependent on the location of the distillery.
Wood or coal can be used to heat a water jacket or directly heat the pot. Typically, these methods are employed for traditional purposes, or due to the lack of utility infrastructure at the distillery, and take even more skill than using an easily-controllable natural gas flame or electricity.
Heating Elements of Stills
Direct Fire is the traditional method of heating a still, where a flame is directly applied to the
pot.
Benefits: This is a quick method of heating a still. Additionally, direct fire is the cheapest method, and can produce different flavors than if the same still and mash/wash were heated with steam, due to the more intense heat. Also, to avoid burning, a rotating chain called a rummager, which resides in the pot and is used to clean the copper, is helpful. Additionally, there are some Geographical Indications that require the use of a direct fire pot still.
Drawbacks: If the distiller is not skilled, this method is more susceptible to burning the contents of the pot or the wash/mash sticking to the inside (making for more difficult cleaning). Additionally, high proof alcohol vapor combined with fire are hazardous. Sticking, however, is not a problem when there are no solids in the pot.
Steam heating consists of using steam that heats a coil or the base of the pot, which, in turn, heats the contents of the still. Steam can also be injected into the still.
Benefits: Faster heating than a water bath. No open flames makes steam less prone to catching on fire.
Drawbacks: Steam can cause dangerous pressure buildups. The production of steam requires additional equipment.
Fluid Bath/Bain Marie Still is similar to using a double boiler on a stove, as the water is heated and acts as a buffer from the direct heat. No water/steam comes into contact with the contents of the pot.
Benefits: The water can also be heated in different ways, including using wood, gas or electricity. It is also a more is a more gentle and forgiving process.
Drawbacks: Bain Marie stills do not reach the high temperature required for a Maillard reaction to occur in the still. This lack of high heat also reduces the ability to drive a reflux type still.
Gas vs. Electric is more a matter of cost and potential environmental impact. The choice is often dependent on the location of the distillery.
Wood or coal can be used to heat a water jacket or directly heat the pot. Typically, these methods are employed for traditional purposes, or due to the lack of utility infrastructure at the distillery, and take even more skill than using an easily-controllable natural gas flame or electricity.
Heating Elements of Stills
Direct Fire is the traditional method of heating a still, where a flame is directly applied to the
pot.
Benefits: This is a quick method of heating a still. Additionally, direct fire is the cheapest method, and can produce different flavors than if the same still and mash/wash were heated with steam, due to the more intense heat. Also, to avoid burning, a rotating chain called a rummager, which resides in the pot and is used to clean the copper, is helpful. Additionally, there are some Geographical Indications that require the use of a direct fire pot still.
Drawbacks: If the distiller is not skilled, this method is more susceptible to burning the contents of the pot or the wash/mash sticking to the inside (making for more difficult cleaning). Additionally, high proof alcohol vapor combined with fire are hazardous. Sticking, however, is not a problem when there are no solids in the pot.
Steam heating consists of using steam that heats a coil or the base of the pot, which, in turn, heats the contents of the still. Steam can also be injected into the still.
Benefits: Faster heating than a water bath. No open flames makes steam less prone to catching on fire.
Drawbacks: Steam can cause dangerous pressure buildups. The production of steam requires additional equipment.
Fluid Bath/Bain Marie Still is similar to using a double boiler on a stove, as the water is heated and acts as a buffer from the direct heat. No water/steam comes into contact with the contents of the pot.
Benefits: The water can also be heated in different ways, including using wood, gas or electricity. It is also a more is a more gentle and forgiving process.
Drawbacks: Bain Marie stills do not reach the high temperature required for a Maillard reaction to occur in the still. This lack of high heat also reduces the ability to drive a reflux type still.
Manipulation of the reactions by the still’s shape and size
The shape of a still influences the amount of reflux, as well as the amount of surface area for copper contact. Generally:
Tall Stills vs. Short Stills
Tall stills encourages reflux and copper reactions as there is more place for copper to interact, and that the steam has to travel further which results in reflux. In short stills the vapor path is easier to traverse therefore less reflux is created.
Wider Neck Stills vs. Narrow Neck Stills
The narrower the stills (swan)neck, the more constricted the vapor path, therefore more reflux occurs. The wider the neck of the still, the less constricted the vapor path, and therefore less reflux occurs.
‘Lamp glass’-shaped stills: Common in Scotch, this shape promotes reflux, but less so than the use of boil bulbs.
For more on the physics of Pot Stills read:
http://cocktailchem.blogspot.com/2014/06/the-physics-of-pot-stills.html
The shape of a still influences the amount of reflux, as well as the amount of surface area for copper contact. Generally:
Tall Stills vs. Short Stills
Tall stills encourages reflux and copper reactions as there is more place for copper to interact, and that the steam has to travel further which results in reflux. In short stills the vapor path is easier to traverse therefore less reflux is created.
Wider Neck Stills vs. Narrow Neck Stills
The narrower the stills (swan)neck, the more constricted the vapor path, therefore more reflux occurs. The wider the neck of the still, the less constricted the vapor path, and therefore less reflux occurs.
‘Lamp glass’-shaped stills: Common in Scotch, this shape promotes reflux, but less so than the use of boil bulbs.
For more on the physics of Pot Stills read:
http://cocktailchem.blogspot.com/2014/06/the-physics-of-pot-stills.html
Manipulation by the Distiller and the Distillation Process
Straining the solids out of the wash before distilling, or distilling the entire wash, including the solids.
The distiller can influence the amount of
reflux by:
Temperature principle: Lowering the temperature of the still.
Concentration Principle: Increasing the ratio of liquid to surface area by putting less liquid into the still.
Allowing air into the still will increase copper’s effect on the distillate and create a lighter spirit, whereas decreasing the amount of air in the still will produce a heavier spirit.
Double distillation instead of single distillation.
Single distillation in a pot still that is not modified by a single or double retort system results in the spirit reaching 40%-60% ABV. Double distillation allows for the resulting spirit to reach 60-80% ABV. In a double distillation setup, the stills may be different than in a single distillation run, because the stripping run is not as high proof.
Manipulating where cuts are made.
Straining the solids out of the wash before distilling, or distilling the entire wash, including the solids.
The distiller can influence the amount of
reflux by:
Temperature principle: Lowering the temperature of the still.
Concentration Principle: Increasing the ratio of liquid to surface area by putting less liquid into the still.
Allowing air into the still will increase copper’s effect on the distillate and create a lighter spirit, whereas decreasing the amount of air in the still will produce a heavier spirit.
Double distillation instead of single distillation.
Single distillation in a pot still that is not modified by a single or double retort system results in the spirit reaching 40%-60% ABV. Double distillation allows for the resulting spirit to reach 60-80% ABV. In a double distillation setup, the stills may be different than in a single distillation run, because the stripping run is not as high proof.
Manipulating where cuts are made.
-
Filipino Still
-
Pot Still with Single Retort
-
Pot Still with Double Retort
<
>
Specific types of Pot Stills
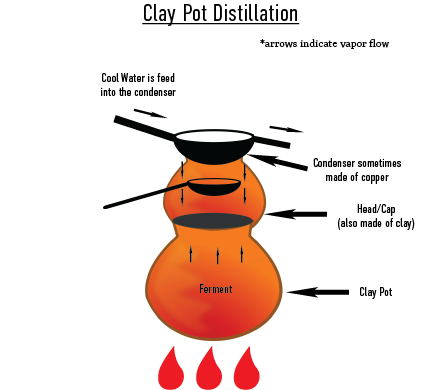
Filipino Still (more generally, East Asian still)
East Asian stills are traditionally made of clay stills. See “Still Materials: Clay” for how these stills are constructed. For historical insight into East Asian stills, read:
Valenzuela-Zapata, Ana Guadalupe. (2014). East Asian Stills. Distillation influences in Mezcal (agave spirits) production in Mexico. https://www.researchgate.net/publication/333194635_East_Asian_Stills_Distillation_influences_in_Mezcal_agave_spirits_production_in_Mexico
Using a clay still:
Filipino Still (more generally, East Asian still)
East Asian stills are traditionally made of clay stills. See “Still Materials: Clay” for how these stills are constructed. For historical insight into East Asian stills, read:
Valenzuela-Zapata, Ana Guadalupe. (2014). East Asian Stills. Distillation influences in Mezcal (agave spirits) production in Mexico. https://www.researchgate.net/publication/333194635_East_Asian_Stills_Distillation_influences_in_Mezcal_agave_spirits_production_in_Mexico
Using a clay still:
- Fermented mash is poured into the still.
- In the production of mezcal, an agave leaf or bamboo tube is placed in the middle of the pot with its tip protruding through the side of the pot in order to create the “parrot.”
- The condenser, a pot of water on the top of the still, is sealed. In mezcal, this is done with a paste made from flour or masa (corn flour) and water.
- A fire is lit underneath the still, and cold water is run over the condenser.
- Most commonly found in the production of ancestral mezcal.
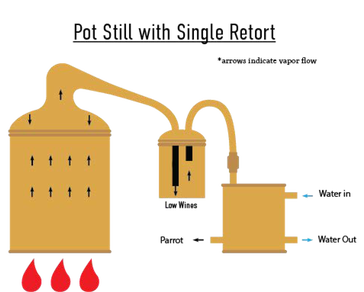
Pot Still with Thumper/Doubler/Single Retort
To increase the efficiency of a distillation by getting a purer final distillate without having to double distill, a second unheated vessel, typically filled with “low wines” (25–35% ABV) from the previous run is attached to the lyne arm of the still. This vessel condenses some of the alcohol vapors into liquid low-wines, and the already liquid low-wines are (re)vaporized by the heat from the incoming vapors. The vaporized alcohol then travels to the condenser. Also:
It can make a thumping sound when filling with vapors, hence the name.
To increase the efficiency of a distillation by getting a purer final distillate without having to double distill, a second unheated vessel, typically filled with “low wines” (25–35% ABV) from the previous run is attached to the lyne arm of the still. This vessel condenses some of the alcohol vapors into liquid low-wines, and the already liquid low-wines are (re)vaporized by the heat from the incoming vapors. The vaporized alcohol then travels to the condenser. Also:
It can make a thumping sound when filling with vapors, hence the name.
- The thumper can be used as a gin basket.
- The thumper can only get as hot as when the ethanol is vaporizing.
- Common in the distillation of: American Whiskey.
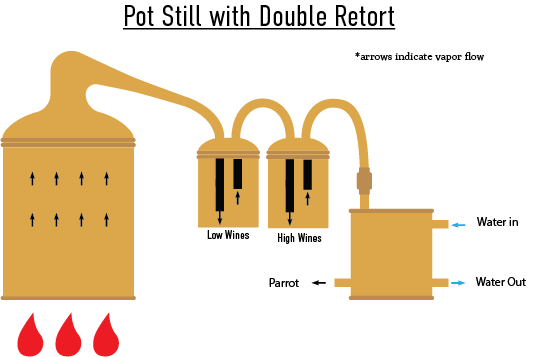
Pot Still with Double Retort
Similar to a single retort in concept, adding an additional retort which is filled with “high wines” (70% ABV approximate) from the previous batch allows for more reflux, resulting in additional purity of the final distillate.
Common in the distillation of: Rum.
For more history and physics of the additional retort, read:
Similar to a single retort in concept, adding an additional retort which is filled with “high wines” (70% ABV approximate) from the previous batch allows for more reflux, resulting in additional purity of the final distillate.
Common in the distillation of: Rum.
For more history and physics of the additional retort, read:
Pot Still with a Reflux Column, aka Hybrid Still
A pot still with either a conjoined column attached to the top of the pot, or adjacent and separate to the pot, and connected via the lyne arm known as “side columns.” Within each column, there are two different approaches to creating reflux: Using packing material or using plates. This still is commonly found in modern craft distilleries because it provides the flexibility of distilling different spirits. It can be run as a pot still by disengaging the columns or engaging one or more reflux columns.
Column with Packing Material
The column is stuffed with removable material which impedes the path of the alcohol vapor and creates reflux. The packing material depends on the type of product the distiller is trying to make, and can be a variety of materials, including stainless steel mesh, copper mesh, ceramic or glass in the form of marbles or Raschig rings. Using packing material is not as controlled as using bubble plates.
Plates (Perforated Plates or Bubble Plates)
Perforated Plates are just that: A metal plate with holes.
Bubble Plates
Bubble plates are perforated, but they have bubble caps that allow some vapor to pass through and the remainder to recondense on the bubble cap. The liquid then drips back down through the column to be vaporized. This high quantity of reflux enables a still using bubble plates to create a much higher proof distillate than other methods of batch distillation.
Turndown ratio: Ratio of minimum vapor load to maximum vapor load
“Downcomer” tube/pipe: Tube that allows the condensed liquid on one plate to flow back down to the next plate or main pot. If side columns are used, there is often a pipe that returns condensed liquid back to the main pot for redistillation.
For more on the physics of batch column stills:
http://cocktailchem.blogspot.com/2019/01/the-physics-of-batch-column-stills-and.html
A pot still with either a conjoined column attached to the top of the pot, or adjacent and separate to the pot, and connected via the lyne arm known as “side columns.” Within each column, there are two different approaches to creating reflux: Using packing material or using plates. This still is commonly found in modern craft distilleries because it provides the flexibility of distilling different spirits. It can be run as a pot still by disengaging the columns or engaging one or more reflux columns.
Column with Packing Material
The column is stuffed with removable material which impedes the path of the alcohol vapor and creates reflux. The packing material depends on the type of product the distiller is trying to make, and can be a variety of materials, including stainless steel mesh, copper mesh, ceramic or glass in the form of marbles or Raschig rings. Using packing material is not as controlled as using bubble plates.
Plates (Perforated Plates or Bubble Plates)
Perforated Plates are just that: A metal plate with holes.
Bubble Plates
Bubble plates are perforated, but they have bubble caps that allow some vapor to pass through and the remainder to recondense on the bubble cap. The liquid then drips back down through the column to be vaporized. This high quantity of reflux enables a still using bubble plates to create a much higher proof distillate than other methods of batch distillation.
Turndown ratio: Ratio of minimum vapor load to maximum vapor load
- Bubble caps have larger turndown ratio
- Perforated plates have smaller turndown ratio
“Downcomer” tube/pipe: Tube that allows the condensed liquid on one plate to flow back down to the next plate or main pot. If side columns are used, there is often a pipe that returns condensed liquid back to the main pot for redistillation.
For more on the physics of batch column stills:
http://cocktailchem.blogspot.com/2019/01/the-physics-of-batch-column-stills-and.html
|
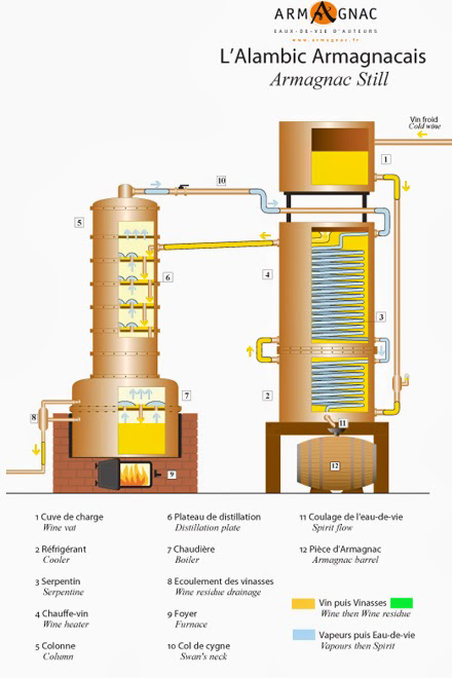
Armagnac Still (Armagnac) [10]
The Armagnac alembic still with reflux column was endorsed in 1818 by a stove maker in Auch, Sieur Tuillière, under the reign of King Louis XVIII. Since then it has been adapted, modified and improved by the region’s distillers. A unique characteristic of the still is that instead of using water to condense the distillate, it uses the undistilled wine to condense the alcohol vapor, which in turn preheats the wine before it enters the still. The wine enters the outside of the heating chauffe-vin (wine heater), then flows into the uppermost plate of the alembic. It then flows down the still until it reaches the boiler. The boiler then heats the wine until the alcohols vaporize. The plates in the column create reflux. Vapors exit through the swan neck and into the condenser, where the wine being fed into the still provides the coolant. |
Vacuum Still
The boiling point of a liquid is dependent on pressure, and as pressure lowers, the boiling point lowers. At sea level, where the pressure in traditional distillation is 1 atm (standard atmosphere), the volatile compounds, including ethanol, vaporize between 170 and 200°F . By reducing the pressure in the still using a vacuum pump, the boiling point will be reduced. The reason this is done is to preserve flavor compounds that may be negatively impacted by heating. The amount of pressure that the still is lowered by depends on the distillery. |
Common in the distillation of: Shochu, gin.
In shochu, pressure is lowered so that alcohol vaporizes at 104-140°F. Oxley Gin, instead of applying heat, lowers the pressure until alcohol vaporizes, which is at the temperature of -5°C. The vapor path leads to a -127°C condenser, which changes the vapor back into a liquid. In this process, no heads or tails are created.
Rotary evaporators are used to vacuum distill small quantities of heat sensitive botanicals which are blended into a pot or column distillate.
For more insight into vapor distillation read:
Pamplin, Bernadette. “Cool Distilling.” Distiller Magazine, 5 Jan. 2021, www.distilling.com/distillermagazine/cool-distilling/ .
In shochu, pressure is lowered so that alcohol vaporizes at 104-140°F. Oxley Gin, instead of applying heat, lowers the pressure until alcohol vaporizes, which is at the temperature of -5°C. The vapor path leads to a -127°C condenser, which changes the vapor back into a liquid. In this process, no heads or tails are created.
Rotary evaporators are used to vacuum distill small quantities of heat sensitive botanicals which are blended into a pot or column distillate.
For more insight into vapor distillation read:
Pamplin, Bernadette. “Cool Distilling.” Distiller Magazine, 5 Jan. 2021, www.distilling.com/distillermagazine/cool-distilling/ .
|
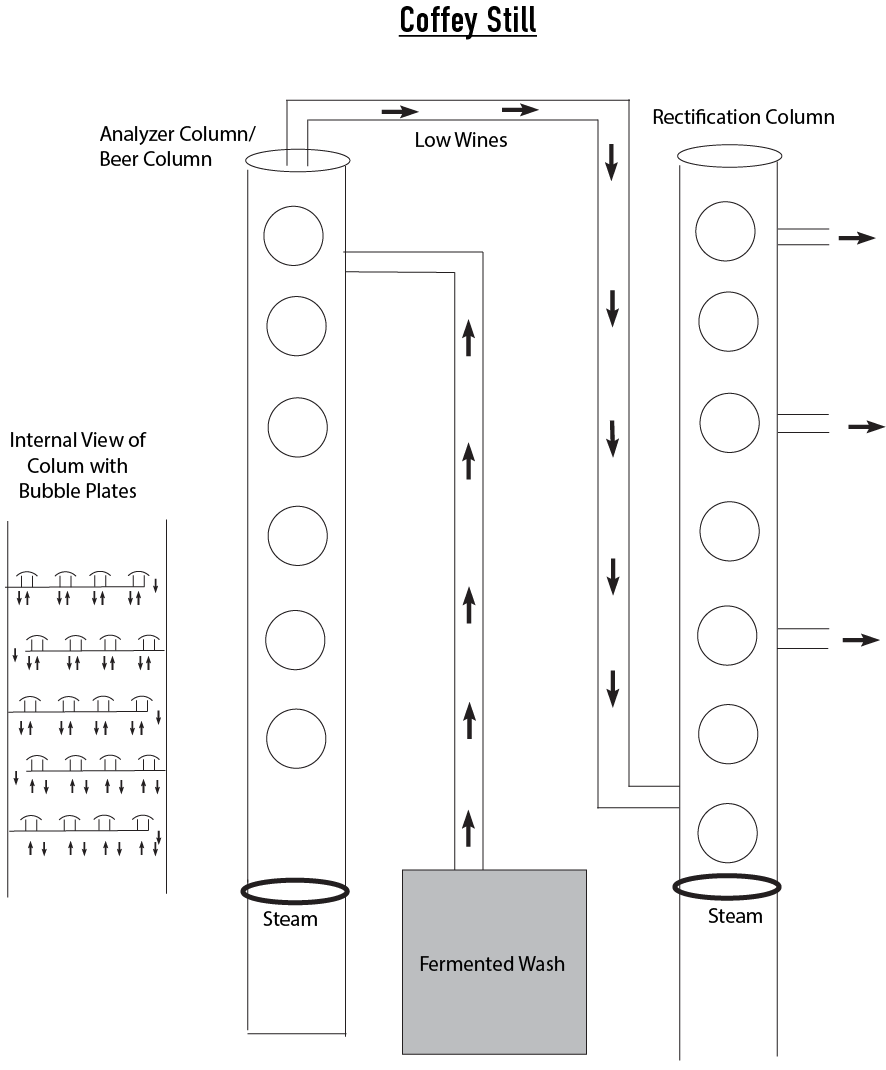
Continuous Distillation in a Fractionating Column
The Coffey Still, though not the first invented continuous still, is the most common. Invented by Aeneas Coffey and then refined by his son Aeneas Coffey, Jr. in the 1840s, each column is similar in construction to a reflux still in that it consists of column(s) and plates. Its goal is to efficiently create consistent high proof distillate (above 95% ABV). In some industrial distilleries, a column can be operated as a set of pre-written parameters with that team member known as an “operators”. These stills are referred to as continuous stills because they can be run 24/7, for as long as there is feedstock to be distilled. The resulting distillate is typically of higher proof than in batch distillation. It should be noted that not all continuous column stills are coffey stills although all coffey stills are continuous column stills. There is also the Kentucky Still. For some history on the Continuous Still read: http://whiskyscience.blogspot.com/2013/08/history-of-column-still.html Types of Fractionating Columns and their Components Traditional Coffey Still The Coffey still is composed of two columns which can be separate or conjoined as one extra tall column. These are: Analyzer column/Beer Column The first column or bottom segment of the column that separates the “low wines” from the mash solids and some of the water. This is equivalent to a stripping run in a pot still. Rectification column The top part of the still or, the second still if the analyzer and rectification columns are separate, is where the low wines are pumped for further refining into the heads, hearts and tails. There is typically plumbing along the different segments of the rectification column so that different cuts can be extracted from the different segments of the column. The rectification column is similar to the spirits run in a pot still. The top of the still is the boiling point of the liquid being selected for (ethanol) The temperature of the column 172° - 190°F (78 - 85°C) at the top of the still to 202° - 212°F (95 - 100°C) at the lowest part of the still depending on the distillery. |
Heating of the column
The vapor for the stripping section is supplied by steam injected at the bottom of the column or by reboiling the liquid from the bottom of the column in the aptly named reboiler. Advantages of a Fractionating Column Higher reflux ratio therefore less energy input and less waste, which means lower costs in the long run. Industrial Still Components Found in large distilleries, a series of column stills can be used to create a more refined spirit. Naturally, larger columns are capable of distilling larger volumes of liquid. However, instead of building a single large vertical column, it can be separated into smaller segments. Steven Cage, President and Owner of Cage and Sons Distilling Systems, provided the following insight into various sub-sections of a distillation column which can also be a column in itself: Aldehyde column is where heads can be collected and sold for industrial usage like fuel ethanol or can be run through another column to further refine them and reclaim usable alcohol. Degassing column can refer to a post rectification process of the hearts portion which removes the higher volatile portion of the heads. Simmering column is used to further refine the "hearts" portion. It is typically used to make high proof, exceptionally neutral alcohol like flavorless vodka or medical grade ethanol. Still Size and Shape Column Height: Should be tall enough to separate all the components. Column height (relative to the diameter) is the most important factor in purity because if the column is too short, insufficient separation will occur. If the column is too tall, more heat is required to increase the speed of vaporization. Column Diameter: Primarily affects the volume of vapor that the column handle. |
Process
Wash is constantly pumped into the top or middle portion of the column where it flows downwards towards the bottom of the still. As it does, it is heated by steam which is injected from the bottom.
Eventually the alcohol gets heated enough to vaporize and travels up the still. As it travels, bubble plates cause reflux.
Alcohol is extracted at the top, the water with the “stillage” remnants accumulating at the bottom.
For the history of the column still read
Strengell, Teemu. History of the Column Still, 1 Aug. 2013,
whiskyscience.blogspot.com/2013/08/history-of-column-still.html.
Wash is constantly pumped into the top or middle portion of the column where it flows downwards towards the bottom of the still. As it does, it is heated by steam which is injected from the bottom.
Eventually the alcohol gets heated enough to vaporize and travels up the still. As it travels, bubble plates cause reflux.
Alcohol is extracted at the top, the water with the “stillage” remnants accumulating at the bottom.
For the history of the column still read
Strengell, Teemu. History of the Column Still, 1 Aug. 2013,
whiskyscience.blogspot.com/2013/08/history-of-column-still.html.
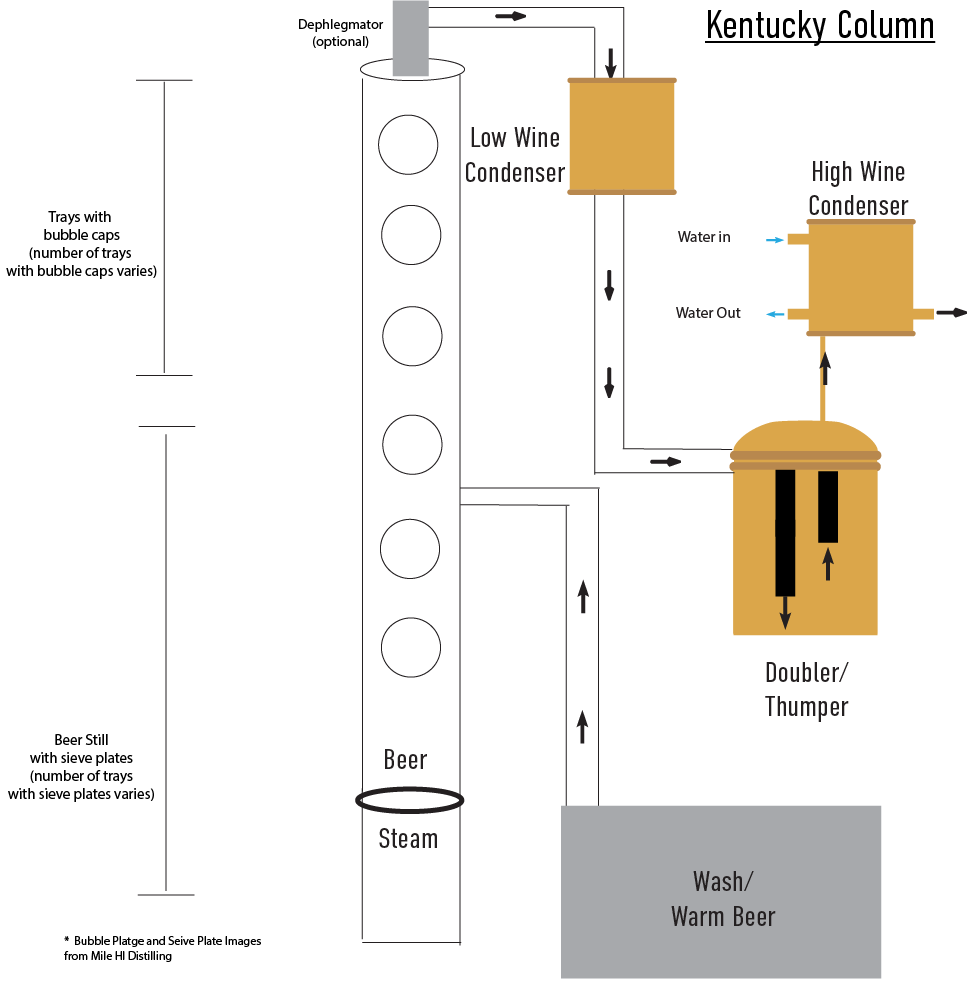
Kentucky Still, aka Kentucky bourbon Still
The Kentucky still, common in midsize to large distilleries, is a modified version of the Coffey still. According to Steven Cage: “While the original Coffey still was designed to produce a mostly neutral spirit, the Kentucky still removed some of this function so as to have a slightly lower proof output (at most 80 proof), and much more flavor. The two designs are more than kissing cousins, they are directly related, taking into account what Coffey learned and published from his initial work.”
Parts
Column still produces low wines
The beer still portion is the bottom portion of the still. The plates of the beer still can be perforated trays which allows for some reflux, but not as much reflux as a bubble plate.
Two to four bubble plates are located towards the top of the still, above where the beer is introduced. If too many bubble plates are used, the distillate will be too high proof to be considered whiskey.
Process 12
The wash is fed into the still above the first sieve tray and begins its downward descent, meeting steam and separating alcohol from water.
The alcohol vapors are often fed into a doubler or a thumper. These are used to redistill the spirit and to provide copper interaction for stills that are not made of copper. The vapor coming out of the doubler or thumper is then led into the condenser, and the resulting distillate is known as 'white dog.'
The 'stillage' is processed into animal feed and 'sour mash,' which is reintroduced into the fermentation process.
The Kentucky still, common in midsize to large distilleries, is a modified version of the Coffey still. According to Steven Cage: “While the original Coffey still was designed to produce a mostly neutral spirit, the Kentucky still removed some of this function so as to have a slightly lower proof output (at most 80 proof), and much more flavor. The two designs are more than kissing cousins, they are directly related, taking into account what Coffey learned and published from his initial work.”
Parts
Column still produces low wines
The beer still portion is the bottom portion of the still. The plates of the beer still can be perforated trays which allows for some reflux, but not as much reflux as a bubble plate.
Two to four bubble plates are located towards the top of the still, above where the beer is introduced. If too many bubble plates are used, the distillate will be too high proof to be considered whiskey.
Process 12
The wash is fed into the still above the first sieve tray and begins its downward descent, meeting steam and separating alcohol from water.
The alcohol vapors are often fed into a doubler or a thumper. These are used to redistill the spirit and to provide copper interaction for stills that are not made of copper. The vapor coming out of the doubler or thumper is then led into the condenser, and the resulting distillate is known as 'white dog.'
The 'stillage' is processed into animal feed and 'sour mash,' which is reintroduced into the fermentation process.
Resources and Suggested Reading
1. Kvaalen, Eric, et al. ALCOHOL DISTILLATION: BASIC PRINCIPLES, EQUIPMENT, PERFORMANCE RELATIONSHIPS, AND SAFETY, Purdue University Cooperative Extension Service, https://www.extension.purdue.edu/extmedia/ae/ae-117.html.
2. United States, Congress, Electronic Code of Federal Regulations. 2020. Https://Www.law.cornell.edu/Cfr/Text/27/5.42 Congress.
3. Porter, E.A. “DISTILLATION.” THERMOPEDIA, 10 Feb. 2011, www.thermopedia.com/.
4 , 9. Morris, Rick. Reflux Still Designs Explained: Part 1, Brewhaus America, 30 Aug. 2017, brewhaus.com/blog/reflux-still-designs-explained-part-1/.
5. “PubChem.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/.
6. Morris, Rick. Reflux Still Designs Explained: Part 1, Brewhaus America, 30 Aug. 2017, www.brewhaus.com/blog/reflux-still-designs-explained-part-1/ .
7. Making Moonshine - The Dummies' Guide, Clawhammer Supply, 11 Feb. 2014, www.clawhammersupply.com/blogs/moonshine-still-blog/12206385-making-moonshine-the-dummies-guide .
8. DragonBlogger. 3 Ways to Manage a Reflux Still Column, Still Dragon, 6 Nov. 2019, stilldragon.com/blog/reflux-still-column/.
10. Bureau National Interprofessionnel De L'Armagnac. “Distillation and Ageing.” Armagnac, www.armagnac.fr/distillation-and-ageing .
11. Ueno, Toshio. “Discovering Shochu: Episode 12 – The Differences between Honkaku Shochu & Korui Shochu.” Episode 12 - The Differences between Honkaku Shochu & Korui Shochu, 31 Oct. 2016, www.sakeschoolofamerica.com/news/discovering-shochu-episode-12-the-differences-between-honkaku-shochu-korui-shochu/ .
12. bishopshomegrown. “The Reason Why Your Whiskey Can and Should Come from Pot Stills, Column Stills, Chamber Stills, Alquitars, Pots with Dephlegmators, Coffee Stills, Pots with Retorts, and Hybrids. Or, How Arguing Pot vs. Column Will Never Broach the Complexity of the Distilling Arts.” The Alchemist Cabinet, 30 Jan. 2019, www.alchemistcabinet.wordpress.com/2019/01/25/the-reason-why-your-whiskey-can-and-should-come-from-pot-stills-column-stills-chamber-stills-alqutars-pots-with-dephlemators-coffee-stills-pots-with-retorts-and-hybrids-or-how-arguing-pot-vs .
Reputable Commercial Still Manufactures
Arnold Holstein: https://a-holstein.de/
Cage and Sons: cageandsons.com
Forsythe Stills: http://forsyths.com/
Vendome Copper and Brass Works Inc.: vendomecopper.com
Additional Reading
For Insight into Choosing a still,
Sherman, Rob. “The Ultimate Guide to Choosing the Right Still for Your Distillery.” The Ultimate Guide to Choosing the Right Still for Your Distillery [BrandScape], Distillery Trail, 31 May 2019, www.distillerytrail.com/resource-library/the-ultimate-guide-to-choosing-the-right-still-for-your-distillery-brandscape/.
Blogs by Distillery Supply Shops
Brewhaus: https://brewhaus.com/blog/
Mile Hi Distilling: https://milehidistilling.com/articles/
Still Dragon: https://stilldragon.com/blog/
Thank you to Steven Cage for the tremendous insight into distilling!
1. Kvaalen, Eric, et al. ALCOHOL DISTILLATION: BASIC PRINCIPLES, EQUIPMENT, PERFORMANCE RELATIONSHIPS, AND SAFETY, Purdue University Cooperative Extension Service, https://www.extension.purdue.edu/extmedia/ae/ae-117.html.
2. United States, Congress, Electronic Code of Federal Regulations. 2020. Https://Www.law.cornell.edu/Cfr/Text/27/5.42 Congress.
3. Porter, E.A. “DISTILLATION.” THERMOPEDIA, 10 Feb. 2011, www.thermopedia.com/.
4 , 9. Morris, Rick. Reflux Still Designs Explained: Part 1, Brewhaus America, 30 Aug. 2017, brewhaus.com/blog/reflux-still-designs-explained-part-1/.
5. “PubChem.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/.
6. Morris, Rick. Reflux Still Designs Explained: Part 1, Brewhaus America, 30 Aug. 2017, www.brewhaus.com/blog/reflux-still-designs-explained-part-1/ .
7. Making Moonshine - The Dummies' Guide, Clawhammer Supply, 11 Feb. 2014, www.clawhammersupply.com/blogs/moonshine-still-blog/12206385-making-moonshine-the-dummies-guide .
8. DragonBlogger. 3 Ways to Manage a Reflux Still Column, Still Dragon, 6 Nov. 2019, stilldragon.com/blog/reflux-still-column/.
10. Bureau National Interprofessionnel De L'Armagnac. “Distillation and Ageing.” Armagnac, www.armagnac.fr/distillation-and-ageing .
11. Ueno, Toshio. “Discovering Shochu: Episode 12 – The Differences between Honkaku Shochu & Korui Shochu.” Episode 12 - The Differences between Honkaku Shochu & Korui Shochu, 31 Oct. 2016, www.sakeschoolofamerica.com/news/discovering-shochu-episode-12-the-differences-between-honkaku-shochu-korui-shochu/ .
12. bishopshomegrown. “The Reason Why Your Whiskey Can and Should Come from Pot Stills, Column Stills, Chamber Stills, Alquitars, Pots with Dephlegmators, Coffee Stills, Pots with Retorts, and Hybrids. Or, How Arguing Pot vs. Column Will Never Broach the Complexity of the Distilling Arts.” The Alchemist Cabinet, 30 Jan. 2019, www.alchemistcabinet.wordpress.com/2019/01/25/the-reason-why-your-whiskey-can-and-should-come-from-pot-stills-column-stills-chamber-stills-alqutars-pots-with-dephlemators-coffee-stills-pots-with-retorts-and-hybrids-or-how-arguing-pot-vs .
Reputable Commercial Still Manufactures
Arnold Holstein: https://a-holstein.de/
Cage and Sons: cageandsons.com
Forsythe Stills: http://forsyths.com/
Vendome Copper and Brass Works Inc.: vendomecopper.com
Additional Reading
For Insight into Choosing a still,
Sherman, Rob. “The Ultimate Guide to Choosing the Right Still for Your Distillery.” The Ultimate Guide to Choosing the Right Still for Your Distillery [BrandScape], Distillery Trail, 31 May 2019, www.distillerytrail.com/resource-library/the-ultimate-guide-to-choosing-the-right-still-for-your-distillery-brandscape/.
Blogs by Distillery Supply Shops
Brewhaus: https://brewhaus.com/blog/
Mile Hi Distilling: https://milehidistilling.com/articles/
Still Dragon: https://stilldragon.com/blog/
Thank you to Steven Cage for the tremendous insight into distilling!